Abstract
Rapid POCT is being used to help address the COVID-19 testing backlog and speed turnaround times. Although the lesser sensitivity and specificity of POCT is acknowledged as an issue, its rapid turnaround time is a worthwhile trade-off in patient scenarios where speed is of the essence. Laboratory professionals are instrumental in ensuring the proper implementation and performance of POCT.
Executive Summary
Point-of-Care Testing (POCT), which has been rising in demand, is even more in the spotlight due to the coronavirus pandemic and the need to quickly act to lessen the virus’ impact. Rapid POCT is a key component of the tools being used to address the need for COVID-19 testing. Although the lesser sensitivity and specificity of POCT is acknowledged as an issue, its rapid turnaround time (TAT) is a worthwhile trade-off in patient scenarios where speed is of the essence.
Key Takeaways
- Early availability of accurate and rapid diagnostic testing is of great value for patient management and public health.
- Development, validation, scale-up, and distribution of diagnostic tests should be a key priority in early preparation during an emerging infectious disease outbreak.
- Multiple testing methodologies and venues, including rapid POCT, are beneficial to meet testing demand and enable contact tracing.
- Laboratory medicine requires an integrated approach.
- Laboratory testing is crucial through all stages of the disease, from diagnosis to epidemiological surveillance.
- Laboratory professionals are well-suited to lead the testing effort in a pandemic and crucial in proper oversight of POCT.
COVID-19 Background & Progression
SARS-CoV-2 is a novel virus that causes the coronavirus disease 2019 (COVID-19). Coronaviruses are a large family of viruses that are typically not harmful to humans. However, there are four known types to cause colds and two that can cause severe lung infections: Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS). The novel coronavirus was named SARS-CoV-2 because of its similarities to the SARS virus.
Even after more than a year of dealing with the coronavirus, our understanding of SARS-CoV-2 and COVID-19 is still evolving and will likely continue to be for several years as we learn the long-term effects of this virus. SARS-CoV-2 is a complex virus that can spread from person-to-person even by people who appear to be asymptomatic, making it much harder to perform contact tracing and manage disease spread. With the first vaccination from Pfizer-BioNTech approved in December of 2020, as of April 2021, we now have vaccinations from Moderna, Johnson & Johnson and AstraZeneca. To date, approximately 19% of the U.S. population has been vaccinated and many treatments are being used with success.
The COVID-19 Situation & Statistics
SARS-CoV-2 is the third serious coronavirus outbreak in less than 20 years, following SARS in 2002-2003 and MERS in 2012. As SARS-CoV-2 can be transmitted person-to-person through respiratory droplets and close contact, a key component of risk mitigation is prevention in high-risk populations. Evidence suggests that vulnerable populations include older adults, people with chronic medical conditions, and those with low immunity. Statistics also continue to show a rise in the number of young people affected.
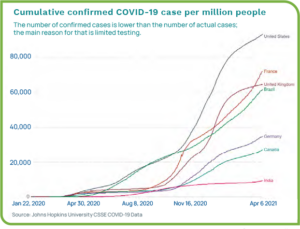
Even as we begin to see light at the end of the tunnel, worldwide overall case numbers are astounding, with the U.S., Brazil, and India dealing with the most infections. Data as of April 2021 shows the total number of confirmed cases globally at more than 132 million, with more than 30.8 million cases in the U.S. Death tolls continue to rise with nearly three million overall and more than 556,000 in the U.S.1 Figure 1 show the number of COVID-19 cases per million for a per-capita look at the statistics.2
COVID-19 is not Comparable to the Seasonal Influenza
SARS-CoV-2 is an unknown, novel virus new to humans, so we still have much to learn to understand how it will affect patients long-term. We have not achieved wide-spread immunity and COVID-19 is significantly more contagious than the flu. In comparison, to put the pandemic into perspective, seasonal influenza kills between 12,000 and 62,000 people annually in the U.S. and between 290,000 and 650,000 people, globally.3 Although there are similarities in transmission and some symptoms, COVID-19 is not the flu. As the death toll rises, COVID-19 has by far killed more people than the seasonal flu does within a similar time span.
1918 Spanish Flu Pandemic
COVID-19 numbers have not reached the devastation of the 1918 influenza outbreak, during which more than one-third of the world’s population (approximately 500 million people) became infected and at least 50 million died worldwide. There are alarming similarities, including the lack of treatments and delay in an effective vaccination because of the virus’ novelty. During the 1918 pandemic, victims died within hours or days of developing symptoms, whereas COVID-19 is not as deadly, but transmission is more difficult to track.
By the summer of 1919, the flu pandemic came to an end, as those that were infected either died or developed immunity. Nearly 90 years later, in 2008, researchers discovered why the flu pandemic was so deadly—a group of three genes enabled the virus to weaken lungs and cause bacterial pneumonia.4
Since 1918, there have been several other viral public health crises, but none as deadly. The novel coronavirus pandemic of 2020 continues to spread around the world as countries race to vaccinate their citizens, develop effective treatments, and struggle with difficult decisions about social distancing and face masks to help avoid spreading the disease.
COVID-19 Timeline & Testing Progression
Right out of the gate, there were struggles with COVID-19 laboratory testing. The list below highlights the progression of the pandemic and associated testing efforts. See Figure 2.
- Initial cases were reported in Wuhan, China in December 2019, with the first case being reported in the U.S. in mid-January.
- As case numbers climbed, on January 31, the WHO declared a global public health emergency.
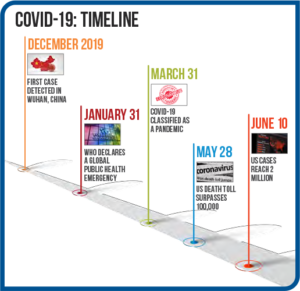
Figure 2: COVID-19 Timeline - Initial testing at the CDC in the U.S. began on February 5, while the WHO was already shipping test kits to non-U.S. public health laboratories. As the CDC began to ship small numbers of test kits to public health labs, problems surfaced due to contaminated reagents, and labs had to ship samples to the CDC for testing.
- U.S. Food and Drug Administration (FDA) rules initially prevented state and commercial labs from developing their own coronavirus diagnostic tests until the FDA enacted the Emergency Use Authorization (EUA) on February 29 that allowed labs to start COVID-19 testing without going through the full FDA approval process.
- In early March, commercial labs in the U.S. began testing and both LabCorp and Quest announced that RT-PCR tests were available. Other labs that are properly equipped to perform biohazardous, highly complex molecular testing began to develop approved tests.
- As cases rose and demand for testing increased rapidly, supplies of collection swabs, reagents, and PPE became strained.
- In late March, the FDA issued an EUA for the first diagnostic POCT.
- In early April, EUAs were issued for the first antibody tests.
- Currently, more than 200 FDA EUA-approved COVID-19 tests are available with varying reliability.
Types of Testing Available for COVID-19
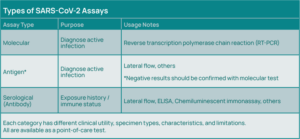
Standard testing for an acute COVID-19 infection uses a molecular testing methodology—reverse transcription polymerase chain reaction (RT-PCR), which involves amplification of viral RNA from respiratory specimens, most commonly nasopharyngeal swabs, but also oropharyngeal swabs, sputum, and bronchoalveolar lavage fluid.
In addition to molecular testing, antigen tests are available that can diagnose active cases of COVID-19, with sensitivity of about 80% relative to PCR. Serology testing is also available to detect viral antibodies (Immunoglobulin M (IgM); Immunoglobulin G (IgG)) (See Figure 3).
Challenges From All Sides
Early bungling of testing efforts gave the virus time to infect thousands of people, and limited testing exacerbated distancing and quarantine efforts. After the FDA relaxed regulations and laboratories had permission to develop tests, testing options began to explode with varying degrees of efficacy.
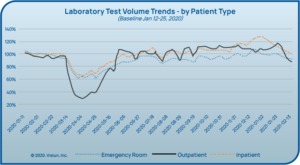
As people were encouraged to stay home and social distance, regular provider visits (and even ED visits) decreased dramatically alongside
laboratory testing volumes in many laboratories (see Figure 4).5 While this testing volume dip has since resolved, laboratories have had to face significant changes. Some laboratories faced furloughs while others were able to reallocate resources to other areas, and many faced shortages of personnel qualified to perform PCR testing. The biggest unforeseen challenge to date has been severe shortages of supplies needed to collect and test for the virus (See Figure 5).6
Supply Chain Worries
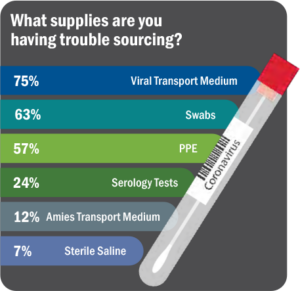
Right from the start, the COVID-19 pandemic has resulted in backlogs and choke points at almost every step of the testing supply chain. Labs either could not get supplies at all, could not get them in the quantities needed to meet volume, or the supplies received were incorrect (See Figure 6).6 To add to the issue of shortages, as new methodologies and platforms are being quickly added, there is a high rate of repeat testing.
“It’s not shortages of any one thing. It’s now spot shortages of all of them,” said Scott Becker, Chief Executive of the Association of Public Health Laboratories. “Clinical labs need more swabs, chemical reagents, viral transport media, test kits, machines to process the tests, and staff to run the machines.”7 Ethical dilemmas and difficult decisions surround the COVID-19 pandemic due to the need to provide urgent care with limited resources and supplies.
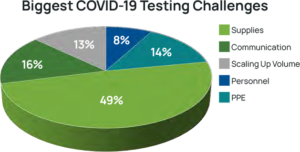
Laboratories Face in COVID-19 Testing8
Reporting Struggles
Initially, laboratories were instructed to report COVID-19 data to their state and local public health laboratories who in turn report data to the CDC. Then, in July, the Coronavirus Aid, Relief, and Economic Security (CARES) Act mandated that “every laboratory that performs or analyzes a test that is intended to detect SARS-CoV-2 or to diagnose a possible case of COVID-19” report the results to the Department of Health and Human Services (HHS) in addition to state or local public health departments “using existing reporting channels …to ensure rapid initiation of case
investigations…”8 Reporting the required data has been hampered by the lack of connectivity and interoperable systems across many laboratories.
It has also been necessary to make pandemic-related workflow modifications to standard electronic lab processes. New processes have had to be designed for:
- Registering, documenting, and printing instrument-ready labels at drive-through testing sites.
- Creating interfaces that exchange test and result information to fill orders from external organizations.
- Sending testing data daily to state DOH and HHS.
Reimbursement Challenges & Government Funding
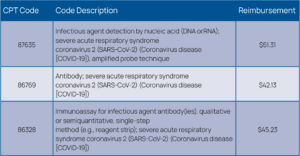
In addition, for many laboratories, reimbursements for COVID-19 testing are only enough to cover the cost of the test kits. Other expenses, such as overhead, salaries, and PPE are not covered by the low reimbursements. Laboratories spend approximately $40 to $150 per test, while CMS reimburses $51 for a standard PCR assay and $100 for a high-throughput test (See Figures 7 and 8).9 PCR testing on instruments can be costly, therefore many laboratories are performing testing in batches, running confirmatory tests wisely to conserve reagents and QC materials and to help keep costs in check.
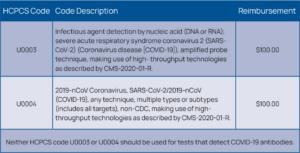
Other COVID-19 reimbursement challenges include the need to verify insurance eligibility and demographic information on patients at emergency testing sites. With the need for rapid TATs, labs have not been delaying testing for billing purposes, which in turn can lead to many claim denials. With higher test volumes, missed charges can have a serious effect on a healthcare organization’s (HCO) finances.10
Variations in Normal Revenue Sources
According to a survey by Kalorama, 59% of laboratory respondents reported significant impact from the COVID-19 pandemic with many initially experiencing declines in almost all testing categories—histology and oncology experiencing the biggest decline. The survey also reported that the greatest concern for laboratories during the pandemic is their people, with about two-thirds concerned about hiring, reducing, and maintaining staff.10
A CAP survey estimated the initial drop in revenues at about 50%, whether or not the lab was offering COVID-19 testing. This decline was attributed to lockdown policies and reduction in scheduled tests and procedures. Additionally, laboratory professionals reported increased stress and burnout.10
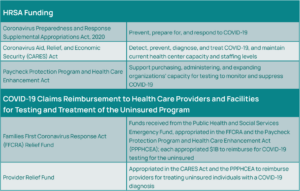
Government Assistance for COVID-19
The Health Resources and Services Administration (HRSA) is awarding approximately $2 billion to HCOs to address COVID-19, including testing and related services (See Figure 9).11 Also, the COVID-19 Claims Reimbursement to Health Care Providers and Facilities for Testing and Treatment of the Uninsured program provides reimbursements directly to eligible providers for claims that are attributed to the testing and treatment of COVID-19 for uninsured individuals (See Figure 9).11
Missed Diagnoses & the Toll on Mental Health
To add to the situation, the COVID-19 pandemic has contributed to an unknown number of missed or delayed diagnoses for non-COVID conditions because patients are unwilling or unable to access medical attention due to fear of COVID-19. For example, HCOs are reporting a higher number of cardiac arrests because patients are waiting too long to seek cardiac care.12
The pandemic has taken a toll on mental health as well with one-third of adults in the U.S. reporting higher stress, anxiety, and sadness. For many, this situation is exacerbated by the threat of unemployment, which has been as high as 16%, comparable to rates experienced during the Great Recession.13
Slow Turnaround Times Delay Contact Tracing
Supply chain bottlenecks and staffing limitations have resulted in longer TATs at a time when a rapid result is imperative to perform contact tracing and slow disease progression. Test results for the novel coronavirus are taking so long that experts say the results are often useless in the campaign to control the deadly disease.
 Several factors continue to increase the demand for testing, such as:
Several factors continue to increase the demand for testing, such as:
- Increasing spread of the virus
- Testing for pre-op patients
- Testing in federally qualified health centers, nursing homes, and prisons
- Orders from drive/walk-through community events
- Organizations bringing employees back to work
- Universities bringing students and faculty to campuses
Some testing sites are struggling to provide results in five to seven days. Others are taking even longer. Long TATs are making it impossible for the U.S. to replicate the strategy used by other countries to effectively contain the virus—test, trace, and isolate.7 Delays in testing mean public healthworkers are not able to notify the contacts of people who test positive. As a result, people continue to spread the virus without knowing they are infected. An additional concern is that slow TATs discourage people from getting tested and practicing social distancing.
Labs Find Ways to Address Challenges & Overcome Barriers
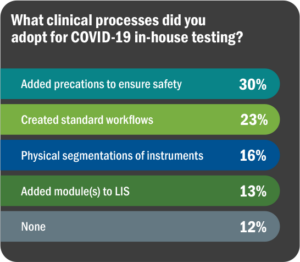
As always, top laboratories find ways to address patient and public health needs, overcoming barriers to the best of their ability. This pandemic has brought on unprecedented challenges, but on the positive side, has resulted in laboratories working together to solve problems while sharing information and education as quickly as possible. Figure 10 lists survey responses regarding adaptive measures taken to bring in COVID-19 testing.
Adopt Multiple Methodologies & Platforms
For example, Central Ohio Primary Care, which performs four million tests annually and operates three patient draw centers, decided that a wise strategy would be to adopt multiple testing platforms to alleviate supply shortage issues and to try to keep up with testing volumes. The laboratory already had the Cepheid GeneXpert in-house, but decided to purchase the DiaSorin Molecular Liaison MDX and the Abbott ID NOW. With a combination of the three platforms, the lab ramped up its capacity to 90 tests per day.9
Add POCT for COVID-19
Many laboratories are turning to POCT to improve TAT. Central Ohio Primary Care added POCT using the Abbott ID NOW system. As mentioned, many POCT options are available for COVID-19 testing, yet concerns have been raised about their lower sensitivity and specificity as well as the need for proper collection techniques.
However, when speed of results is vital, such as in a worldwide pandemic, POCT becomes imperative. Even a less-sensitive result is way better than taking forehead temperatures in a doorway as people enter the building. Herein lies an opportunity for laboratory professionals to explain the proper use of POCT and ensure that samples are being collected and results are being interpreted appropriately.

Diligently Track Reimbursements
In addition, to ensure appropriate reimbursement, laboratory leaders should stay in close communication with operations, billing, and IT to make sure that the appropriate revenue for testing is being captured. This can involve steps such as capturing accurate demographic information, utilizing the proper billing codes, and tracking payments from government and commercial payers.
“This [tracking of reimbursements] needs to be a robust effort because guidelines are changing and practices are changing. If that is not done, the lab will find that COVID-19 testing will lead to a very significant reduction in their operating margin,” explains David Nichols, President and Founder of Nichols Management Group.6
Spotlight on the Value of POCT
Rapid POCT is being introduced to help address the testing backlog and delayed TATs. Although the lesser sensitivity and specificity of POCT is pointed out as a flaw, its rapid TAT is a worthwhile trade-off in patient scenarios where speed is of the essence.
 POCT for Vulnerable Populations
POCT for Vulnerable Populations
HHS has begun distributing FDA-authorized POCT diagnostic devices to nursing homes in COVID-19 hotspot areas to facilitate on-site COVID-19 testing for patients and staff. This effort is intended to allow nursing homes to augment their current capacity for testing and improve their ability to quickly respond and prevent disease spread. “Access to rapid point-of-care testing in nursing homes will further protect our Nation’s most vulnerable patients,” said ADM Brett P. Giroir, M.D., Assistant Secretary for Health.14
Also, in an effort to protect vulnerable populations, HHS has awarded $760 million to purchase the Abbott BinaxNOW COVID-19 Ag Card, which costs approximately five dollars per test and provides results in about 15 minutes. The test includes a smartphone app that allows patients to receive their test results.15
The Laboratory’s Evolving Role
The rapid spread of COVID-19 across the world has exposed major gaps in the abilities of most countries to respond to a virulent new pathogen. Moving forward, as we work to control the COVID-19 pandemic and plan for future pandemics, a key lesson is that early availability of diagnostic testing is of great value for patient management and public health. Thus, the development, validation, scale-up in manufacturing, and distribution of diagnostic tests should be a key priority in early preparation during an emerging infectious disease outbreak.
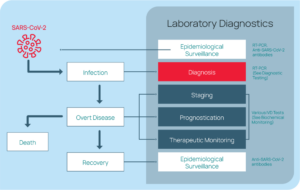
Rapid, reliable testing is crucial to understanding the spread of the pandemic and responding appropriately. Even after initial diagnosis, laboratory testing is important through all stages of patient care (i.e., disease staging, prognosis, treatment plan guidance, and epidemiological
surveillance). See Figure 11.16
Although POCT is imperfect, when performed as intended, its rapid TAT is a worthwhile trade-off in certain patient care situations. POCT can be an effective part of an integrated testing approach. Laboratory professionals who have training and knowledge about testing methodologies and limitations are well-suited to help determine when and where POCT is appropriate and to help ensure that testing is being performed accurately. Medical laboratory scientists should embrace POCT as a powerful tool in the right places when speed is critical and utilize their knowledge of POCT as an opportunity to showcase their contribution to the healthcare team.
Orchard Software’s POCT Software Solution
Orchard Software offers Point-of-Care Testing (POCT) management and integration software that allows POC coordinators to manage their POCT program from a central location, addressing the challenges of remote POCT oversight. This means easy tracking of disparate devices across multiple locations, tracking operator competency assessments for hundreds to thousands of end users per device, interfacing and communicating with disparate POCT devices, and ensuring that care gaps are closed and POCT are automatically billed as the testing is completed. In addition, Orchard’s POCT solution is vendor-agnostic, includes decision-support rules that improve laboratory productivity, and can help standardize POCT across locations. Orchard’s POCT solution stands out from its competitors because its implementation is flexible enough to improve POCT workflows across many different types of healthcare organizations.
Orchard Point-of-Care Testing Network
Orchard Software is proud to announce its Point-of-Care Testing Network intended to help businesses integrate and connect point-of-care testing across multiple geographic locations, including rapid point-of-care testing for COVID-19. The Orchard Point-of-Care Testing Network can improve processes associated with point-of-care testing for any business that intends to track point-of-care test results, including skilled nursing and senior living facilities, nursing homes, educational institutions, airlines, etc.
The Orchard Point-of-Care Testing Network enables electronic test ordering and automated result reporting as well as automatic reflex ordering where indicated. In addition, the Point-of-Care Testing Network supports various government reporting requirements and provides integration to HHS, state health departments, and the Centers for Disease Control and Prevention (CDC). The solution will enable facilities to manage point-of-care testing devices from a central location, track point-of-care testing patient orders and results, meet CLIA requirements for point-of-care testing, and compile and report required data.
Reference List
- COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html?fbclid=IwAR1aXn4rDVMVzshmJkmlFWh0196aKv7vKIQnnypsOpt4lInXe6VujuzveRk#/bda7594740fd40299423467b48e9ecf6. Updated April 7, 2021. Accessed April 7, 2021.
- Our World in Data. Statistics and Research. Coronavirus (COVID-19) Cases. https://ourworldindata.org/covid-cases#daily-confirmed-cases-per-million-people. Accessed April 7, 2021.
- Disease Burden of Influenza. Centers for Disease Control and Prevention. https://www.cdc.gov/flu/about/burden/index.html. Updated October 5, 2020. Accessed April 7, 2021.
- Spanish Flu. History.com. https://www.history.com/topics/world-war-i/1918-flu-pandemic. Updated August 11, 2020. Accessed April 7, 2021.
- COVID-19 Business Intelligence and Analysis for Clinical Laboratories, Pathology Groups and Hospital Administration. https://covid19briefings.com/visiun-2/. Updated March 9, 2021. Accessed April 7, 2021.
- Wilson, L. COVID-19 testing, despite supply chain issues. MLO. https://www.mlo-online.com/disease/infectious-disease/article/21142733/covid19-testing-despite-supply-chain-issues. June 24, 2020. Accessed April 7, 2021.
- Weiner, R., Wan, W., Hauslohner, A. Long delays in getting test results hobble coronavirus response. The Washington Post. https://www.washingtonpost.com/health/long-delays-in-getting-test-results-hobblecoronavirus-response/2020/07/12/d32f7fa8-c1fe-11ea-b4f6-cb39cd8940fb_story.html. Published July 12, 2020. Accessed April 7, 2021.
- HHS Announces New Laboratory Data Reporting Guidance for COVID-19 Testing. HHS.gov. https://www.hhs.gov/about/news/2020/06/04/hhs-announces-new-laboratory-data-reporting-guidance-for-covid19-testing.html. June 4, 2020. Accessed April 7, 2021.
- Wilson, L. COVID-19 testing and the revenue cycle. MLO. https://www.mlo-online.com/management/rcm/article/21138310/covid19-testing-and-the-revenue-cycle. May 20, 2020. Accessed April 7, 2021.
- Hayes, E. Labs absorb revenue losses, adapt to a ‘new normal.’ LabPulse.com. https://www.labpulse.com/index.aspx?sec=sup&sub=mic&pag=dis&ItemID=801182. May 12, 2020. Accessed April 7, 2021.
- Health Resources & Services Administration. Coronavirus (COVID-19) Information. https://www.hrsa.gov/coronavirus. Updated February 2021. Accessed April 7, 2021.
- Carr, S. Missed and Delayed Diagnoses of Non-COVID Conditions — Collateral Harm from a Pandemic. Society to Improve Diagnosis in Medicine. https://www.improvediagnosis.org/improvedx-newsletter/improvedx-july-2020/missed-and-delayed-diagnoses-of-non-covid-conditions-collateral-harm-from-apandemic/. July 2020. Accessed April 7, 2021.
- Kochhar, R. Unemployment rose higher in three months of COVID-19 than it did in two years of the Great Recession. Pew Research Center. https://www.pewresearch.org/fact-tank/2020/06/11/unemploymentrose-higher-in-three-months-of-covid-19-than-it-did-in-two-years-of-the-great-recession/. June 11, 2020. Accessed April 7, 2021.
- Trump Administration Announces Initiative for More and Faster COVID-19 Testing in Nursing Homes. https://www.hhs.gov/about/news/2020/07/14/trump-administration-announces-initiative-more-fastercovid-19-testing-nursing-homes.html. Published July 14, 2020. Accessed April 7, 2021.
- Trump Administration Will Deploy 150 Million Rapid Tests in 2020. https://www.hhs.gov/about/news/2020/08/27/trump-administration-will-deploy-150-million-rapid-tests-in-2020.html. Published August 27, 2020. Accessed April 7, 2021.
- Lippi, G., Plebani, M. The critical role of laboratory medicine during coronavirus disease 2019 (COVID-19) and other viral outbreaks. Clin Chem Lab Med. 2020 Jun 25;58(7):1063-1069. doi: 10.1515/cclm-2020-0240. PMID: 32191623. Accessed April 7, 2021.
