Abstract
Point-of-care testing (POCT) technology continues to advance at a fast pace, making it easier to shrink complex testing platforms into point-of-care (POC) devices. By testing at a molecular level, the accuracy of the results far surpasses previous POCT methodologies. Molecular POCT devices allow quick, easy-to-use testing in near-patient scenarios where a rapid diagnosis can be the difference between treating one patient and treating all others who would be infected if the diagnosis were delayed.
Key Takeaways
- Access to sensitive and rapid infectious disease assays is essential for accurate diagnosis, effective treatment, and timely infection control.
- Innovations in molecular POCT have allowed molecular testing to expand from specialty molecular diagnostics laboratories into clinical laboratories, clinics, and at the patient’s side.
- The rapid turnaround time (TAT) of POCT combined with superior sensitivity and specificity of molecular methodologies affords the best scenario for diagnosis and containment of contagious infectious diseases.
- For public health emergencies, molecular POCT can be used to achieve accurate, economical diagnoses, promoting early detection and mitigation to better control an infectious disease outbreak.
Molecular Testing Overview
Molecular testing is a broad term that refers to the detection and/or quantification of specific DNA or RNA sequences in a specimen. Molecular tests are used to detect microorganisms, look for genetic mutations associated with certain diseases and cancers, perform paternity tests, and much more. Highly complex molecular testing is performed in molecular or microbiology specialty laboratories by specialty trained laboratory professionals.
Traditional Molecular Testing—Highly Accurate, Long TAT
 The starting principle behind molecular testing involves multiplying (or amplifying) the amount of DNA within a specimen thousands to millions of times prior to analyzing. DNA is amplified using cycling methods, such as polymerase chain reaction (PCR) or isothermal amplification. This means that samples with only a few hundred infectious particles can be amplified billions of times, thereby increasing the likelihood of detection, and potentially compensating for suboptimal sample collection.
The starting principle behind molecular testing involves multiplying (or amplifying) the amount of DNA within a specimen thousands to millions of times prior to analyzing. DNA is amplified using cycling methods, such as polymerase chain reaction (PCR) or isothermal amplification. This means that samples with only a few hundred infectious particles can be amplified billions of times, thereby increasing the likelihood of detection, and potentially compensating for suboptimal sample collection.
The amplification of DNA/nucleic acids is achieved using naturally occurring enzymes (proteins). DNA polymerase is one of the key enzymes used in this process, which is the basis for the PCR methodology. Other enzymes can be used for amplification and these are typically grouped under the generic label of isothermal nucleic acid amplification tests (INAAT). The amplification step makes these testing methodologies more sensitive than immunological assays. PCR testing involves amplification achieved through temperature cycling, whereas isothermal amplification assays use a constant reaction temperature for the amplification that results in a shorter runtime than PCR.
The high level of reliability and accuracy that molecular testing techniques offer typically comes with a longer TAT—attributed to batch testing, complex multi step testing, and predetermined testing schedules. Traditional molecular diagnostic methodologies require sophisticated infrastructure, expensive reagents, stable electrical power, and highly skilled staff.
Frequency of Testing & TAT
Molecular diagnostic tests can be divided into two groups based on the frequency of testing:
- One-time: Testing to detect the presence of mutations or specific sequences in the human genome that are associated with certain diseases only needs to occur once in a patient’s lifetime. These tests typically do not require a rapid TAT.
- Ongoing: Diagnostic assays for infectious diseases, or genetic mutations associated with drug metabolism or a tumor need to occur more frequently to assess patient status. Because infectious diseases are contagious and can be life-threatening, rapid TAT is imperative to enable appropriate treatment.
Molecular POCT Market Outlook
From a technology stance, the molecular POCT diagnostics market splits into three categories:
- Polymerase chain reaction (PCR)
- Isothermal nucleic acid amplification technology (INAAT)
- Other technologies (microfluidic technology and microarrays)
Data from 2019 shows an increasing number of respiratory infectious diseases contributing to the growth of the respiratory diseases market segment, which comprises the largest share of the molecular POCT market.1 The POC molecular diagnostics market is expected to grow at a compound annual growth rate of 13.2% from 2019 to 2027, reaching $2.4 billion. This growth rate is driven by the rising prevalence of chronic diseases, development of CLIA-waived molecular POC tests, venture capital funding for molecular POCT development, lack of skilled professionals, and the need for rapid decision making in emergency departments.1
Infectious Disease Testing
During the last three decades, more than 30 new infectious diseases have been identified, making it imperative to have a reliable method to identify the responsible pathogens.2 Access to sensitive and rapid infectious disease assays is essential for accurate diagnosis, effective treatment, and timely infection control.
Non-molecular POCT—Rapid TAT, Lower Sensitivity
 POCT allows for rapid diagnosis and subsequent timely treatment. Rapid, easy-to-use POCT has long been used to detect infectious disease antigens or antibodies, such as influenza, mononucleosis, and group A Streptococcus (GAS). However, despite offering a quick TAT, many of these assays come with a sensitivity and specificity trade-off that is less than ideal. Conventional, nonmolecular POCT can have suboptimal limits of detection resulting in false negatives for samples with low viral or bacterial load. In fact, for influenza, a negative antigen test is routinely reflexed to a confirmatory molecular test due to the high prevalence of false negatives.
POCT allows for rapid diagnosis and subsequent timely treatment. Rapid, easy-to-use POCT has long been used to detect infectious disease antigens or antibodies, such as influenza, mononucleosis, and group A Streptococcus (GAS). However, despite offering a quick TAT, many of these assays come with a sensitivity and specificity trade-off that is less than ideal. Conventional, nonmolecular POCT can have suboptimal limits of detection resulting in false negatives for samples with low viral or bacterial load. In fact, for influenza, a negative antigen test is routinely reflexed to a confirmatory molecular test due to the high prevalence of false negatives.
Similarly, diagnosis of GAS infections in pediatric patients is greatly enhanced by rapid, accurate testing. Although antigen-based POC tests offer a quick TAT, the testing has a relatively low sensitivity and specificity (compared to culture) and can be difficult to interpret, potentially leading to erroneous results. As such, the recommendation is to reflex negative results to a confirmatory molecular method.3
Advantages of Molecular POCT for Infectious Disease
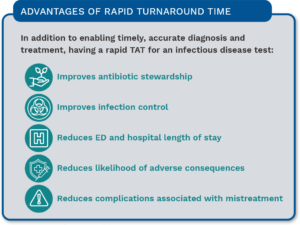
In contrast, molecular POCT for GAS provides rapid and more accurate results that enable faster diagnosis and treatment, eliminating the time and cost for confirmatory testing. By offering accurate testing at the patient side, results can be acted upon in a timelier manner. Figure 1 lists advantages afforded by a rapid TAT.
Improved Precision & Accuracy
Molecular POCT is much faster than lab-based molecular tests and more accurate than existing non-molecular POCT. The precision and accuracy of molecular POCT gives clinics or other smaller healthcare centers the ability to deliver a standard of care equivalent to a tertiary care center.4
Addressing the Skill Shortage
Another factor driving the demand for molecular POCT is the scarcity of skilled laboratory professionals available to perform traditional molecular testing. Molecular POCT allows clinics to perform testing that previously could only be accomplished with specialized staff.
Antibiotic Stewardship
In addition, the use of more sensitive and specific molecular tests at the POC promotes more appropriate use of antibiotics.
Available Molecular POCT
Many traditional molecular instrument vendors now offer molecular POCT. Rapid, CLIA-waived molecular POCT is currently available for influenza, respiratory syncytial virus (RSV), GAS, and an array of respiratory pathogens. Figure 2 outlines a list of available molecular POCT by methodology.
Molecular POC Tests for COVID-19

Response to the COVID-19 pandemic has also been challenged by long delays associated with centralized laboratory PCR testing. In hospitals, these delays lead to poor patient flow and nosocomial transmission.
The rapid, accurate test results that molecular POCT methodologies offer are extremely beneficial in a public health crisis, such as a pandemic. Molecular POCT is associated with faster results, improvements in infection control measures, and better patient flow as compared with centralized laboratory PCR testing.5 A rapid, accurate, low-cost diagnostic POC device is imperative for timely diagnosis and mitigation of COVID-19 and future pandemics.6 Figure 3 lists available molecular POCT for SARS-CoV-2/COVID-19.
For public health emergencies, molecular POCT can be used to achieve accurate, economical diagnoses, promoting early detection and mitigation to better control an infectious disease outbreak. A rapid, accurate, cost-effective device benefits individual patients and the public by reducing transportation needs, lowering the spread of infection, and reducing the cost of testing.7

Orchard Software Solutions
Orchard Software Corporation is a leader in the laboratory information system industry and offers a variety of laboratory system solutions, including a SaaS model. Orchard serves more than 2,000 laboratories across the country, helping them improve efficiency, reduce errors, and enhance integration. Orchard’s products are installed in physician groups and clinics, hospitals, independent reference labs, student health centers, veterinary labs, public health organizations, universities, and retail facilities.
Orchard Molecular
Orchard Molecular includes dynamic workflow support that orchestrates the entire molecular testing process from pre-analytical through post-analytical. Our molecular solution is part of a comprehensive and integrated enterprise solution that includes clinical, pathology, microbiology, toxicology, outreach, and point-of-care testing functionality. Our systems capture data in a searchable, integrated format and offer a flexible implementation, allowing Orchard to meet the connectivity, workflow, and reporting needs of any size molecular laboratory. The solution allows rapid adoption of diverse molecular diagnostics, including support for biochemical genetics, carrier screening, cytogenetics, infectious disease testing, prenatal testing, and newborn screening.
Orchard Point-of-Care
Orchard® Point-of-Care™ offers centralized governance of a comprehensive POCT program across patient testing locations. The solution includes support for specimen collection, routing, and tracking processes, device management and integration, and a feature-rich competency module. POC coordinators can track patient testing, devices, operator certifications, and QC from a central location to help ensure quality testing and promote patient safety.
References
- Meticulous Market Research Pvt. Ltd. Point of Care (POC) Molecular Diagnostics Market Worth $2.39 Billion by 2027, Growing at a CAGR of 13.2% from 2019- Pre and Post COVID-19 Market Opportunity Analysis and Industry Forecasts by Meticulous Research® June 22, 2020. Accessed at http://www.globenewswire.com/news-release/2020/06/22/2051397/0/en/Point-of-Care-POC-Molecular-Diagnostics-Market-Worth-2-39-Billion-by-2027-Growing-at-a-CAGR-of-13-2-from-2019-Pre-and-Post-COVID-19-Market-OpportunityAnalysis-and-Industry-Forecas.html
- Hansen, S. & Wahed, A. Point-Of-Care or Point-Of-Need Diagnostic Tests: Time to Change Outbreak Investigation and Pathogen Detection. Tropical Medicine and Infectious Disease. September 2020. Accessed at http://www.mdpi.com/2414-6366/5/4/151/pdf
- Larkin, P. & Garner, O. Molecular Point-of-Care Testing in Clinical Laboratories. CLN. July 2020. Accessed at http://www.aacc.org/cln/articles/2020/july/molecular-point-of-care-testing-in-clinical-laboratories
- Jacobs, E. Molecular Diagnostics & Point of Care Testing [Webinar]. Accessed at https://www.whitehatcom.com/POCWebMtgs/Slides/E_Jacobs_Molecular_Technology_in_POC_052721.pdf
- Brendish, N.J., et. al. Clinical impact of molecular point-of-care testing for suspected COVID-19 in hospital (COV-19POC): A prospective, interventional, non-randomised, controlled study. The Lancet. October 2020. DOI: https://doi.org/10.1016/S2213-2600(20)30454-9
- Green, K., et. al. Molecular and antibody point-of-care tests to support the screening, diagnosis and monitoring of COVID-19. The Centre for Evidence-Based Medicine. April 2020. https://www.cebm.net/covid-19/molecular-and-antibody-point-of-care-tests-to-support-the-screening-diagnosis-andmonitoring-of-covid-19/
- Ahmad, S., Ali, N., Kausar, M., Misbah, H., & Wahid, A. Road toward rapid-molecular point of care test to detect novel SARS-coronavirus 2019 (COVID-19): Review from updated literature. Allergol Immunopathol (Madr). 2020;48(5):518-520. https://dx.doi.org/10.1016%2Fj.aller.2020.06.001
