Cervical cancer is one of the leading causes of cancer deaths among women; yet, with proper screening tests, it can be diagnosed early and successfully treated.
 Due to advances in molecular testing, a DNA amplification test to detect human papillomavirus (HPV)—the virus closely associated to cervical cancer—is now available. With this testing capability comes new recommendations for cervical cancer screening. Laboratories can increase their contribution to patient care by being aware of these guidelines and using laboratory information system (LIS) tools to help track and support compliance.
Due to advances in molecular testing, a DNA amplification test to detect human papillomavirus (HPV)—the virus closely associated to cervical cancer—is now available. With this testing capability comes new recommendations for cervical cancer screening. Laboratories can increase their contribution to patient care by being aware of these guidelines and using laboratory information system (LIS) tools to help track and support compliance.
Executive Summary
- Early diagnosis of cervical cancer greatly improves the chances of successful treatment.
- More than 99% of cervical cancers are related to the HPV infections.
- Laboratories are key players in providing screening testing that can prevent cervical cancer.
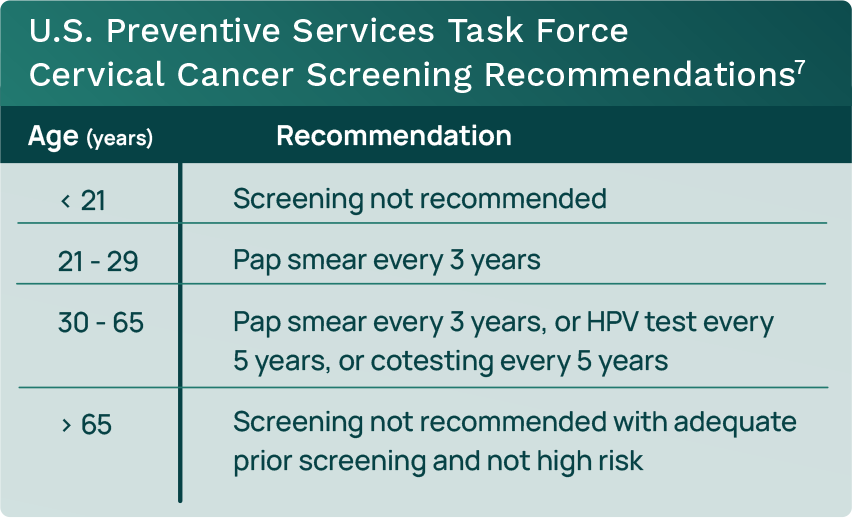
- Current screening protocols recommend cotesting (Pap smear and HPV) for women aged 30 to 65.
- Cytology-specific LIS tools help labs identify patients who need screening according to the current recommendations.
Lab Testing for Women’s Health
Some health issues, such as cervical cancer, are specific to women and require periodic laboratory screening tests. By proactively screening for female-related health issues, healthcare providers can identify health risks earlier and prevent more serious problems. In addition to the standard laboratory testing for a wellness check (e.g., complete blood count, comprehensive metabolic panel, lipid panel), experts recommend periodic testing for cervical cancer.
Cervical Cancer
Cervical cancer is the fourth most frequently diagnosed cancer and fourth most common cause of cancer-related mortality among women. In 2020, worldwide, more than half a million women contracted cervical cancer, and nearly 350,000 died.1
More than 99% of cervical cancers are due to HPV, the most common reproductive tract viral infection. Two HPV types, specifically 16 and 18, are responsible for about half of cervical pre-cancers. Most sexually active people are infected with HPV at some point in their lives, but 90% can clear the infection without symptoms or treatment. However, all women with HPV are at risk for pre-cancerous lesions that can progress to cervical cancer.
Cervical Cancer Screening
When detected early, cervical cancer has one of the most successful recovery rates. Screening protocols can be lifesaving because testing can lead to early diagnosis and treatment. As screening tools, the Pap smear shows abnormal cervical cells, and the HPV test shows if a patient has a type of HPV that is known to cause cervical cancer.
Pap Smear
Used for decades, the most common test performed for cervical cancer screening is the Pap smear. The test gets its name from the doctor who first discovered its use as a screening tool for cervical cancer, Georgios Papanicolaou.2 A Pap smear is a cytology test that involves collecting and examining cervical wall cells for abnormalities that may become cancerous if not treated. For cervical cancer screening, a Pap smear can be performed as a standalone test or combined with an HPV test.
Human Papillomavirus (HPV)
 More recently, recommendations were released that include a test for HPV depending on the patient’s age and other risk factors. The HPV test looks for DNA from HPV in cervical cells. Often, HPV infections are asymptomatic and clear up without treatment; however, persistent infections with specific oncogenic HPV types are expected to progress to cancer. There are about 14 high-risk HPV types including HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68.3 As mentioned, HPV 16 and 18 are responsible for most HPV-related cancers. Primary high-risk human papillomavirus (hrHPV) testing can be performed without the Pap smear using a test that is FDA-approved for standalone screening.4
More recently, recommendations were released that include a test for HPV depending on the patient’s age and other risk factors. The HPV test looks for DNA from HPV in cervical cells. Often, HPV infections are asymptomatic and clear up without treatment; however, persistent infections with specific oncogenic HPV types are expected to progress to cancer. There are about 14 high-risk HPV types including HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68.3 As mentioned, HPV 16 and 18 are responsible for most HPV-related cancers. Primary high-risk human papillomavirus (hrHPV) testing can be performed without the Pap smear using a test that is FDA-approved for standalone screening.4
In 2020, due to studies demonstrating the greater efficacy of hrHPV testing, the American Cancer Society updated cervical cancer screening guidelines to recommend hrHPV testing as the preferred screening method. However, implementation of this guideline was initially slow because of the limited availability of FDA-approved HPV tests and the laboratory infrastructure changes required to implement. In the future, it is expected that the Pap smear will be phased out, replaced by hrHPV testing as testing becomes more accessible. The Pap may remain as an option in cervical cancer screening guidelines.3
Cotesting
Cervical cancer testing that includes both a Pap smear and an HPV test is called “cotesting,” and has been identified as the best way to reduce the incidence of cervical cancer and related mortality. By incorporating HPV testing with the Pap test, there is a greater chance of early detection when compared to the standalone Pap smear. Because of this finding, current guidelines recommend three screening options:
- Pap smear only
- hrHPV testing only
- Cotesting5
Screening Recommendation Details
Clinical trial data show that for average-risk patients aged 25 to 65, primary hrHPV testing and cotesting detect more cases of abnormal cervical cells than cytology alone, but hrHPV-based tests are associated with an increased risk of false-positive results.6
The current U.S. Preventive Services Task Force (USPSTF) screening recommendations include both Pap smear and HPV testing (see Table 1).
How the Lab Can Help
Laboratories are key players in providing screening testing that can identify pre-cancerous cells. Using LIS data mining and decision-support tools specific to cytology, labs can identify patients who are due for screening and associate that with the specific screening recommendations for the patients’ ages. The LIS can generate lists that are age- and gender-specific to determine which testing is appropriate according to current recommendations. With the ability to track age-specific recommendations for testing, laboratories can pinpoint the patients needing screening, then generate follow-up letters as screening test reminders.
Orchard Enterprise Pathology Offers Cytology-specific Tools
Orchard® Enterprise Pathology™ is part of Orchard Software’s enterprise solution that enables enterprise-level scalability, flexibility, security, and reliability. The solution offers tools that have been developed specifically to support cytology testing and to help laboratories satisfy diagnostic guidelines for women’s health.
Summary
Cervical cancer screening options have expanded, and cervical cytology (Pap smears), primary hrHPV testing, and co-testing are all currently part of an effective screening program to detect cervical cancer. Laboratories can use their LIS tools to be an effective part of identifying and tracking screening testing needs for women’s health.
References
- World Health Organization. Cervical cancer. www.who.int/news-room/fact-sheets/detail/cervical-cancer. Published February 2022.
- Tan SY, Tatsumura Y. George Papanicolaou (1883-1962): discoverer of the Pap smear. Singapore Med J. 2015 Oct;56(10):586-7. doi: 10.11622/smedj.2015155. PMID: 26512152; PMCID: PMC4613936.
- National Cancer Institute. HPV and cancer. www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-and-cancer. Published April 2023.
- American College of Obstetricians and Gynecologists Practice Advisory. Updated cervical cancer screening guidelines. www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2021/04/updated-cervical-cancer-screening-guidelines. Published April 2021.
- Terasawa T, et al. Comparative accuracy of cervical cancer screening strategies in healthy asymptomatic women: a systematic review and network meta analysis. Sci Rep 12, 94. https://doi.org/10.1038/s41598-021-04201-y. Published 2022.
- Andersen B, et al. HrHPV testing vs liquid-based cytology in cervical cancer screening among women aged 50 and older: a prospective study. International Journal of Gynecologic Cancer. 2020;30:1678-1683. https://ijgc.bmj.com/content/30/11/1678. Published 2020.
- U.S. Preventive Task Force. Cervical cancer: screening. www.uspreventiveservicestaskforce.org/uspstf/draft-update-summary/cervical-cancer-screening-adults-adolescents. Published March 2022.

