Every reputable laboratory is focused on quality. All laboratories have a quality assurance system in place to ensure accurate results and continuous quality improvement (CQI). Tracking quality is often broken into the steps of the total testing process (TTP): pre-analytical, analytical, and post-analytical. By evaluating every step of the TTP, labs reduce errors and enable valuable medical decision making and effective patient care.
A laboratory’s greatest tool to both track and prevent errors is its laboratory information system (LIS). The LIS can support a QA program that covers the TTP with quality measures that are tracked for each area.
Pre-analytical Errors
Most errors occur in the pre-analytical phase, with estimates that this phase accounts for up to 70% of laboratory errors.1 Included in the pre-analytical phase, and also error-prone, are errors associated with procedures performed outside the laboratory walls by healthcare personnel not under the direct control of the clinical laboratory. Pre-analytical errors include problems during patient preparation, sample collection, transportation, and sample preparation.
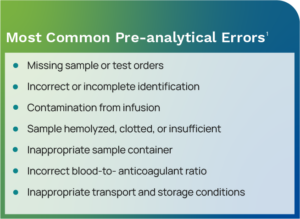
How Your LIS Helps Reduce Pre-analytical Errors
Because pre-analytical errors are the most common, the LIS includes a multitude of functionality that supports error reduction for this phase. Tools like decision support rules standardize the specimen ordering and processing procedure, removing the opportunity for errors. There are LIS rules for test routing, split specimens, insurance verification, bar code labels, collection tube information, messaging, duplicate tests, patient match, collection notes, and more.
Analytical Errors
In an era of staffing shortages with staff often working long hours, staff fatigue can cause mistakes. Leverage your LIS to automate as many processes as possible to reduce errors related to repetitive tasks. In the analytical phase, errors can occur when procedures are not standardized or not followed or when steps for preventive maintenance and/or QC are not completed.

How Your LIS Helps Reduce Analytical Errors
The biggest way that your LIS supports the analytical phase of testing is through connectivity—connecting to the EHR to receive orders, connecting to the analyzers to relay specimen information and orders, and then sending results back to the EHR. An integrated LIS ensures that the lab is part of an interoperable healthcare organization and reduces errors by automating the orders and results processes.
The LIS can also be used to set up formulas, auto-verify “normal” results, track QC and linearity studies, and capture instrument flags to guide laboratory scientists to investigate an issue. Checklists can be created that track instrument maintenance and ensure instruments are ready for sample testing.
Post-analytical Errors
Even at the post-analytical phase, errors still occur. Data entry errors can arise when results are recorded manually. In some instances, verbal information may be passed on incorrectly or misheard. These errors happen after the test is conducted, and include failure in reporting, erroneous data validation, improper data entry, and excessive turnaround time.

How Your LIS Helps Reduce Post-analytical Errors
Using the LIS’s bar code technology can help improve and maintain high levels of QA at the post-analytical phase. Bar coded specimens prevent specimen misidentification and automate results delivery to the proper chart and provider. Digital transmission of reports ensures that results are received in a timely manner to allow for timely medical decisions.
Your LIS can help you develop a troubleshooting plan by which you identify and track errors to prevent a recurrence. The LIS can help you establish a standard for result ordering and reporting, reducing errors significantly throughout the TTP.
Leverage Your LIS to:
- Drive CQI
- Enhance your analytical process
- Reduce laboratory errors
- Standardize processes
- Optimize data recording and storage
- Save time to focus on important tasks
- Track samples through each testing phase
- Implement successful validation protocols
- Support documentation for inspection and proficiency testing
References
- Plebani M. Quality indicators to detect pre-analytical errors in laboratory testing. Clin Biochem Rev. 2012 Aug;33(3):85-8. PMID: 22930602; PMCID: PMC3428256.
- Abdollahi A, Saffar H, Saffar H. Types and frequency of errors during different phases of testing at a clinical medical laboratory of a teaching hospital in Tehran, Iran. N Am J Med Sci. 2014 May;6(5):224-8. doi: 10.4103/1947-2714.132941.
