Abstract
Myriad changes within the U.S. healthcare system are pressuring laboratory professionals to update their thinking, focusing on a broader scope of laboratory stewardship and becoming cognizant of the value of the laboratory’s contribution in addition to quality lab result reporting. Laboratories are encouraged to find specific new ways to contribute in a value-based, patient-centered healthcare system, thereby broadening their impact, proving their worth within their organization, and ensuring stability and sustainability.
Focus on Laboratory Stewardship
The Laboratory’s Evolving Value Proposal
Legislation and advances in technology are aligning to reshape how healthcare is delivered in the United States. The restructuring of the U.S. healthcare system has already begun to have an influence on the laboratory market. With legislation such as the Protecting Access to Medicare Act of 2014 (PAMA) and the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA), laboratories need to understand that they are in a position to contribute to value-based care and overall cost reductions in healthcare, and now is the time to do so. In fact, some laboratories are already learning to leverage their patient data to make positive contributions to patient care. Many close to the industry believe it is of vital importance for laboratories to clearly demonstrate their value in this new paradigm as the industry shifts and decisions are made regarding whether to maintain an internal lab or to outsource lab testing.
Changes in Healthcare
Although there has been major discussion of repealing the Affordable Care Act (ACA), as well as disagreement between governmental parties regarding the best direction for the U.S. healthcare system, there is widespread bipartisan agreement that regardless of the exact trajectory, overall change must continue down the path to value-based care. The ACA started the shift to value-based reimbursements and began the rise of Patient-centered Medical Homes (PCMHs) and Accountable Care Organizations (ACOs). As of first quarter 2017, ACOs in the United States totaled more than 900, impacting delivery of healthcare for more than 32 million patients.1 In addition, other Advanced Alternative Payment Models (APMs) are gaining traction, with the shift continuing beyond shared-savings to include shared-risk and fully capitated payments. The Health Care Payment Learning & Action Network (LAN) reported in 2016 that 29% of healthcare payments were associated with APMs (up from 23% in 2015), and the LAN anticipates 50% of payments will be within APMs by the end of 2018.2 Furthermore, the shift to value-based payment models is being reinforced by the revamping of provider reimbursements via replacement of the Medicare Sustainable Growth Rate (SGR) formula and consolidation of several quality programs into one—the Quality Payment Program (QPP).
MACRA’s Quality Payment Program: MIPS & APMs
The QPP was published in October 2016 by the Centers for Medicare & Medicaid Services (CMS) as part of MACRA. The QPP is a value-based reimbursement system that has two options:
- Merit-based Incentive Payment System (MIPS)
- Advanced Alternative Payment Models (Advanced APMs)
Providers can select which path they want to take in the QPP based on their practice size; specialty, location, or patient population; and their level of participation in value-based models. Providers who bill more than $90,000 a year to Medicare or treat a minimum of 200 Medicare patients are subject to MIPS payment guidelines. MIPS participants will be compared with other providers and benchmarking standards. The data from 2017, the first year of performance tracking, was used to determine 2019 payment adjustments.
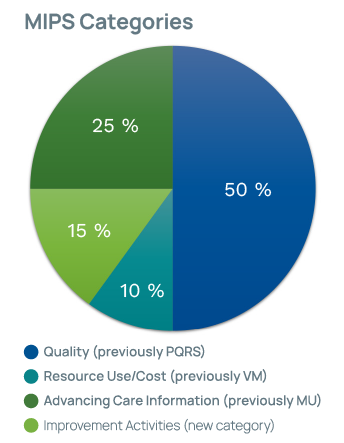 Once MIPS is in full swing, providers will be evaluated for four categories:
Once MIPS is in full swing, providers will be evaluated for four categories:
- Quality Measures (replaces Physician Quality Reporting System [PQRS])
- Cost (replaces Value Modifier [VM] program)
- Advancing Care Information (ACI) (replaces EHR Incentive Programs/Meaningful Use [MU])
- Improvement Activities (new category)
In 2019, physicians will receive a score that will result in a potential adjustment (up or down) to Medicare Part B reimbursements. Medicare payment adjustments for providers range from +/-4% starting in 2019 to +/-9% in 2022.
Advanced APMs offer greater possible rewards for taking on greater risks. Organizations that participate through an Advanced APM can earn a 5% incentive bonus on Part B reimbursements and are exempt from MIPS. Eligible Advanced APMs involve downside risk, quality measurements, and health information technology (HIT) requirements. They are meant for providers already participating in specific value-based care models.
Rise of the Engaged Patient
Another healthcare reform-related movement is increased awareness of the need for greater patient engagement. Patient engagement has been referred to as “the blockbuster drug of the century” because of its potential to improve the status of overall health.3 Whereas strategic planning in the business sector has always taken the consumer perspective into account, healthcare has been slower to catch onto the concept. Patients need to be involved and held accountable for their choices and actions, and have a better understanding of their health issues. As the value of patient engagement is becoming more widely recognized, simultaneously, the industry is developing more HIT tools to support this movement. In addition, people are beginning to expect to be connected and have electronic access to healthcare as they do in other industries. In fact, one of every 20 Google searches relates to a health question.3 However, the challenge is getting over the hurdle to where everyone (patients and healthcare workers) understands and expects patients to be engaged.
The first level of patient engagement is simply shared decision-making, where the provider lays out various treatment options and the patient can make an educated choice as to which decision fits their belief system and specific personal needs—weighing benefits and risks to come to the best individual option.4 The next level of patient engagement is to have a patient who is “activated,” which refers to a specific “willingness” to be more involved in one’s health. Several studies have shown that activated patients see better outcomes and spend less on healthcare; specifically, lower levels of patient activation resulted in up to 21% higher costs.4
 Changes in the Laboratory Market
Changes in the Laboratory Market
The revamping of the U.S. healthcare system has spurred an increase in mergers and consolidations among healthcare organizations (HCOs) and within the laboratory industry as facilities restructure to gain economy of scale. For laboratories, this can shift where testing takes place. Yet there is no denying that the backbone of diagnostics resides within the laboratory and that providers rely on laboratory testing for diagnosis and treatment planning. Therefore, savvy laboratories are poised to contribute to value-based care and overall cost reduction in healthcare. In fact, some laboratories are already learning to leverage their patient data to make positive contributions to patient care. It behooves those in the laboratory to demonstrate the lab’s value in this new healthcare arena.

PAMA’s Impact on Laboratory Reimbursements
One of the biggest lab industry upsets of all time is PAMA, which mandates that the CMS establish market-based laboratory reimbursement rates. The PAMA Final Rule, published in June 2016, sparked apprehension across the industry. Part of the controversy is that “applicable labs” that were required to report their payer reimbursements to determine the new pricing were from a very limited portion of the market. Applicable labs were defined as those receiving more than 50% of Medicare revenue from the Clinical Laboratory Fee Schedule (CLFS) or Physician Fee Schedule (PFS), with an annual minimum of $12,500 in reimbursements. This definition eliminated almost all hospital labs and resulted in new pricing being based on less than 1% of the total laboratory market.5 Additionally, it has been suggested that an onerous process for data submission resulted in incomplete and inaccurate data, further skewing the market-pricing determination.6 These factors put many in the industry up in arms over the slanted data used for the updated fee schedule and raised concerns over the effect on patient care if lab access decreases in certain areas.
In September 2017, the CMS published the newly proposed CLFS, with significant cuts slated for many of the high-volume routine tests (e.g., chemistry, immunochemistry, and hematology). In all, 75% of tests on the CLFS are reduced as a result of the market-based pricing scheme.7 As a result, labs that are running with a low profit margin and whose organizations are not involved in value-based reimbursements will feel the biggest impact. In particular, labs in rural areas with high volumes of Medicare patients will experience a greater percentage of cuts. There are predictions that many smaller, independently owned labs will come under the umbrella of the two largest reference laboratories, LabCorp and Quest, in order to survive. These two big reference labs currently hold 27% of the lab market share.8
An updated estimate from the CMS predicts that PAMA cuts will result in Medicare Part B reductions of around $670 million for 2018. In addition, for labs whose testing menu consists mainly of the top 20 highest-volume tests, reimbursements for this test group are expected to be cut by an average of 28% by 2020.6 Concurrently, reimbursements for molecular tests are expected to increase, paving the way for price increases for proprietary genetic and molecular tests, such as Myriad Genetics’ Vectra DA increasing by 42% to $840.65.9
Decentralized Lab Testing
Even prior to PAMA, the industry experienced a significant number of mergers and consolidations, both among HCOs and within the laboratory market, because smaller facilities cannot afford to stay open in the new market. Results of a Chi Solutions survey indicate that 34% of lab outreach programs have been approached with an offer of purchase or merge, and many are taking these offers seriously.8
With the threat of what PAMA offers, hospitals and other HCOs are expected to take the new pricing into consideration in their long-term planning. PAMA demands that healthcare facilities re-evaluate whether or not they plan to stay in the laboratory business. While some see the laboratory as central to their mission and like to have control of their valuable laboratory data, others may consider outsourcing to a large reference lab. The question is, what exactly pushes this decision in one direction or the other?
 Opportunities for the Lab
Opportunities for the Lab
The silver lining is that change brings new opportunities. Remarkably, laboratory expenses are only about 3% of the overall healthcare spend, yet lab results contribute to nearly every patient’s diagnosis or treatment plan. Furthermore, the high rate of diagnostic error can be attributed in part to misutilization of laboratory tests.10 A recent study found that up to 75% of cases evaluated indicate errors in diagnostic testing.10 The high usage of lab testing and its rate of misuse opens a window of opportunity for laboratory professionals to become more involved in decisions about which tests are ordered and how they are interpreted. In this time where it is incumbent upon laboratories to show their value, this opportunity should be taken seriously.
Lab Formularies: A First Step
Recently, there have been efforts to monitor and control laboratory test utilization through lab formularies and other utilization monitoring initiatives, such as cascade testing and EHR notifications. A lab formulary can be developed to reduce or eliminate ordering of expensive esoteric tests or to make sure that when there are options, the best test is selected. Some laboratories use a tiered system where certain tests can only be ordered by specialists without pathologist or lab director approval. In any case, development of a lab formulary requires collaboration with providers and ongoing modifications to keep the requirements in line with the most recent evidence-based recommendations.12 The downside is that typically the lab formulary is more focused on reducing costs than on ensuring that the best tests are ordered, which leaves room for improvement.

 Laboratory Stewardship: A Goal
Laboratory Stewardship: A Goal
The concept of laboratory stewardship has been introduced to take the lab formulary to the next level. A laboratory stewardship program focuses on making sure the best tests are ordered as well as properly interpreted, with an eye on patient safety. Historically, laboratories have focused on efficiency; however, the “practice of laboratory medicine” includes involvement in downstream healthcare processes related to lab testing.14 This creates an opportunity for laboratorians to become actively involved with providers in determining best test selection, performance, and interpretation. Laboratory stewardship means the laboratory takes responsibility for and is active in use of lab testing, from order through interpretation to analytics. Key elements include governance, interventions, data extraction and monitoring, and ongoing improvement strategies.15
 As the move to value-based reimbursement progresses and the shift toward capitated payments continues, lab stewardship programs will become invaluable resources to ensure proper use of lab testing and to develop diagnostic teams that are a collaboration between laboratory professionals and providers.15 Standardizing laboratory practices using evidence-based guidelines can improve efficiency, patient satisfaction, quality of care, and overall clinical operations.16 Furthermore, because the laboratory serves a large portion of patients, the effects of a diagnostic or laboratory stewardship program can have a broader reach than that of an antibiotic stewardship program.17
As the move to value-based reimbursement progresses and the shift toward capitated payments continues, lab stewardship programs will become invaluable resources to ensure proper use of lab testing and to develop diagnostic teams that are a collaboration between laboratory professionals and providers.15 Standardizing laboratory practices using evidence-based guidelines can improve efficiency, patient satisfaction, quality of care, and overall clinical operations.16 Furthermore, because the laboratory serves a large portion of patients, the effects of a diagnostic or laboratory stewardship program can have a broader reach than that of an antibiotic stewardship program.17
Introducing the Diagnostic Management Team (DMT)
Dr. Michael Laposata has been touting the value of a diagnostic management team (DMT) for decades, and the move to value-based care is helping this concept gain traction. In 2017, the first DMT conference took place in Galveston, Texas, and was quite well-received. The concept of a DMT involves a group of laboratory medicine experts that reviews test orders and results and offers specific narratives to the provider to guide interpretation and future testing.18 This type of intervention has become necessary because as the number of diagnostic tests continues to rise rapidly, the amount of time physicians spend learning about laboratory testing in medical school pales in comparison.
 In fact, the rate of incorrect diagnosis has been reported as high as one in every 10 patients.18 This problem snowballs when an unnecessary test has abnormal results that are irrelevant to the situation, but it has added costs, patient stress, and, potentially, more unnecessary procedures.
In fact, the rate of incorrect diagnosis has been reported as high as one in every 10 patients.18 This problem snowballs when an unnecessary test has abnormal results that are irrelevant to the situation, but it has added costs, patient stress, and, potentially, more unnecessary procedures.
With the burgeoning complexity of lab test options, physicians are open to experts’ guidance on best test selection and interpretation. The laboratorian and the provider both want the best outcome for their patients. However, in a fee-for-service healthcare model, expert advice regarding lab orders and results is not reimbursable, leaving us with very few diagnostic experts who can guide physician lab orders.18 Dr. Laposata has had great success with the DMT initiative and its ability to support providers in test interpretation and to make a real difference in patient outcomes.
The Move toward Population Health Management
Historically, the U.S. healthcare system has focused on individual patients in siloed care sites; however, to address rapidly escalating healthcare costs and improve the quality of care, population health management (PHM) is being considered an instrumental part of the transition to value-based care. Laboratories must understand and find ways to be involved in PHM to thrive in the evolving healthcare environment.
 As an example, Central Ohio Primary Care (COPC) is actively pursuing PHM and the move to value-based care by effectively implementing care teams and
As an example, Central Ohio Primary Care (COPC) is actively pursuing PHM and the move to value-based care by effectively implementing care teams and
handling care interventions, and its laboratory is completely on board. Members of its care team meet with high-risk patients to assess their needs. The care team nurse is responsible for medication reconciliation, follow-up concerns, next appointments, and communication with the rest of the care team. The most critical patients receive home care visits with the goal of providing whatever palliative or hospice care necessary to allow elderly patients to stay in their homes.19
 COPC’s lab continues to find ways to add value and is actively supporting its PHM initiatives. The laboratory is involved in efforts such as:
COPC’s lab continues to find ways to add value and is actively supporting its PHM initiatives. The laboratory is involved in efforts such as:
- Finding new sources of revenue (e.g., wellness screens and on-site employer health centers)
- Supporting population health initiatives (e.g., primary care efforts for millennials)
- Working with other ancillaries (e.g., diagnostic test scheduling to support emergent or next-morning testing)
- Supporting continuity of care (e.g., open later hours and weekends)
- Using lab data to help providers meet MIPS quality measures (contribute to all specialties represented)
POCT: Another Window of Opportunity
As laboratory budgets shift in response to PAMA and value-based models, laboratory menus will shift as well. Careful consideration should be given to where point-of care testing (POCT) can be of value. A renewed focus on patient satisfaction and patient-centered care, as well as a more holistic view of the patient’s needs, shines a new light on the potential for POCT. Even though POCT’s cost per test may be more than the cost of that test in a core lab, if implemented in a scenario where the rapid turnaround time that POCT offers creates significant downstream savings, the cost is well justified. For instance, D-dimer testing to screen for pulmonary embolism or troponin testing to screen for myocardial infarction in an urgent care setting can reduce unnecessary admissions or decrease a length of stay. In scenarios like these, the cost of the POCT is far overshadowed by the downstream savings.


 In value-based healthcare, it is time to look at POCT in regards to impact on patient outcomes rather than at cost per test. The cost of POCT will always be greater than core lab testing because of automation and economies of scale; however, by looking at the savings generated, the benefit of POCT is clearly demonstrated.20 With value-based care, the demand for POCT will continue to rise, offering an opportunity for laboratory professionals who have expertise in lab testing to manage POCT so that maximum value is obtained.
In value-based healthcare, it is time to look at POCT in regards to impact on patient outcomes rather than at cost per test. The cost of POCT will always be greater than core lab testing because of automation and economies of scale; however, by looking at the savings generated, the benefit of POCT is clearly demonstrated.20 With value-based care, the demand for POCT will continue to rise, offering an opportunity for laboratory professionals who have expertise in lab testing to manage POCT so that maximum value is obtained.
 Looking to the Future: Genetic Testing
Looking to the Future: Genetic Testing
In addition to POCT, molecular diagnostics is revolutionizing healthcare by paving the way for tailored treatments at the individual level—
personalized medicine. Precision medicine and personalized medicine go hand in hand with companion diagnostics, which are genetic and molecular laboratory tests used to determine a patient’s benefits and risks with taking certain medications. Lab testing has steadily moved from a very hands-on process to a more automated process. The majority of large laboratories now include automation lines that handle the routing of samples, and automation is expanding its reach into microbiology, anatomic pathology, and molecular testing. Furthermore, providers are demanding additional support in the interpretation of genetic test results. The role of the laboratorian is expected to become more consultative. In addition, the lab can be involved in using software solutions to sort through and analyze the large amounts of data generated by genetic testing. We are looking at a future where IT systems can analyze databases with genome analyses from hundreds of thousands of patients for trends or connections that aid in determining the best treatment plan for a particular patient. This lends to the thought that the future role of the laboratorian will shift away from specimen handling toward data processing and interpretive guidance.
With IT advances, molecular and genetic testing advances, and a changing healthcare environment, the lab is transitioning away from hands-on processes to a reality where the specimen is rarely touched. What the laboratorian will “touch” is the data—being involved in valuable interpretations, test consultations and algorithms, and lab informatics. Laboratory professionals can engage in active education with other healthcare colleagues to promote the integration of molecular advances into routine patient care, as well as aid in molecular and genetic data analysis and result interpretation to realize the full benefit of molecular testing and promote maximum lab value.
Lab 2.0
A collaborative group of innovative laboratories—Project Santa Fe—is pushing the needle toward demonstrating lab value. Its recent publication explains the need for labs to “upgrade” from Lab 1.0 (transactional) to Lab 2.0 (integrative) in line with the healthcare terrain.11 The group touts that laboratories, through engaging in leadership opportunities that integrate the laboratory in PHM and patient care improvements, can be instrumental in identification of at-risk patients and tracking of patient progress.11 In addition, laboratorians can collaborate with clinicians about risks and care gaps, evidence-based guidelines, and help guide clinical decisions based on test results. In order to accomplish this paradigm shift, laboratory professionals must understand how lab data relates to clinical outcomes and cost of care throughout the entire care continuum. The idea is for labs to become aware of the downstream costs or cost savings associated with lab test results, and to realize that lab results can impact more than their 3% of the healthcare budget.

Change Management/Culture
Many of us cling to old familiar ways because there is comfort in familiarity; change can be stressful because you do not know what to expect. One of the most difficult endeavors within HCOs is managing change and creating a culture that is adaptable and “change-ready.” This concept has gained importance in light of the rapid changes taking place and the need to shift to a sustainable system focused on better patient outcomes.
 New Ways to Add Value
New Ways to Add Value
There is logic in aligning healthcare payments with quality and value. It also makes sense for patients to become active members of their own care team, held accountable for their choices. Advances in technology, laboratory medicine, HIT, and mobile technologies offer new ways to interact with patients, and these advances change patients’ expectations for care interactions. As these developments culminate, laboratory professionals need to understand the bigger picture and realize that lab results influence many moving parts outside the four walls of the laboratory. Understanding and acknowledging the value that lab data has downstream is a key step. There is no doubt that lab testing is vital in patient care; however, the future of healthcare calls for laboratorians to enhance the contribution of lab results by assistance in proper test order selection and interpretation, PHM and preventive care initiatives, and other collaborative projects that use lab analytics to save downstream healthcare dollars.
Call to Action
Begin to process the concept of laboratory stewardship and Lab 2.0 and think about what happens downstream in your facility based on reported lab results. Think about lab testing from its beginning to end, including well before the pre-analytical phase and well beyond the post-analytical phase, and determine how your laboratory is fully leveraging its lab testing to improve care and reduce costs.
 Here are some questions to consider:
Here are some questions to consider:
- Does your lab offer the most appropriate testing menu and hours of operation for your patient population?
- Are there opportunities for POCT to reduce TAT or improve patient satisfaction that have not been considered?
- Are there molecular tests that can aid in certain patient populations that your lab services?
- Are there opportunities for preventive testing that can be helpful to your patient population?
- How can your lab support PHM and risk stratification of patients?
- Are there MACRA quality areas that your lab can support?
- Do you have physician or pathologist champions who are open to discuss a DMT?
In summary, the lab is an incredibly valuable service line that has tremendous potential to add value in the new healthcare model. Make sure your lab shines in value-based care.

References
- Muhlestein, D., Saunders, R., & McClellan, M. (2017, June). Growth of ACOs and alternative payment models in 2017. Health Affairs. Retrieved from http://www.healthaffairs.org/do/10.1377/hblog20170628.060719/full/
- Barnett, J. C., & Berchick, E. R. (2017, September). Census.gov. Retrieved from https://www.census.gov/content/dam/Census/library/publications/2017/demo/p60-260.pdf
- Foisey, C. Q. (2015, October). The importance of patient engagement and technology. Health IT Outcomes. Retrieved from https://www.healthitoutcomes.com/doc/the-importance-of-patient-engagement-andtechnology-0001
- James, J. (2013). Patient engagement. Health Affairs Health Policy Brief. http://dx.doi.org/10.1377/hpb20130214.898775
- Philippidis, A. (2017, December 14). Clinical lab group sues HHS over proposed PAMA 2018 Medicare rates. Clinical Omics. Retrieved from https://clinicalomics.com/articles/clinical-lab-group-sues-hhs-overproposed-pama-2018-medicare-rates/1396
- Michel, R. (2017, October). For top 20 tests, CMS to cut payment by 28% in 2018-2020. The Dark Report. Retrieved from http://www.darkreport.com
- McCurdy, D. A., & Daubert, G. L. (2017, September 26). Medicare clinical lab fee schedule payments to fall by $670 million in 2018 under preliminary PAMA rates. Reed Smith. Retrieved from https://www.healthindustrywashingtonwatch.com/2017/09/articles/other-health-policy-developments/other-cms-developments/medicare-clinical-lab-fee-schedule-payments-to-fall-by-670-million-in-2018-underpreliminary-pama-rates/
- Fong, T. (2017, May). The PAMA effect: Consolidation of clinical labs expected as legislation set to take effect. 360Dx. Retrieved from https://www.360dx.com/regulatory-news/pama-effect-consolidationclinical-labs-expected-legislation-set-take-effect
- Klip, J., Kaufman, J., & Scott, K. (Eds.). (2017, October). C21 members to get rate hikes under proposed CLFS for 2018. Laboratory Economics. Retrieved from http://www.laboratoryeconomics.com
- Sarkar, M. K., Botz, C. M., & Laposata, M. (2017). An assessment of overutilization and underutilization of laboratory tests by expert physicians in the evaluation of patients for bleeding and thrombotic disorders in clinical context and in real time. Diagnosis2017, 4(1), 21–26. http://dx.doi.org/10.1515/dx-2016-0042
- Crawford, J. M., Shotorbani, K., Sharma, G., Crossey, M., Kothari, T., Lorey, T.S., …Fisher, N. (2017). Improving American healthcare through “clinical lab 2.0”: A project Santa Fe report. Academic Pathology, 4, 1-8. http://dx.doi. org/10.1177/2374289517701067
- Lewandrowski, K., & Sluss, P. M. (Eds.). (2017). Utilization management in the clinical laboratory and other ancillary services. https://doi.org/10.1007/978-3-319-34199-6
- Dickerson, J. A., Fletcher, A. H., Procop, G., Keren, D. F., Singh, I. R., Garcia, J. J., …Astion, M. L. (2017). Transforming laboratory utilization review into laboratory stewardship: Guidelines by the PLUGS National Committee for Laboratory Stewardship. The Journal of Applied Laboratory Medicine, 2(4). http://dx.doi.org/10.1373/jalm.2017.023606
