The value-focused environment of modern healthcare industry is increasing the demand for rapid, accurate, and integrated point-of-care testing (POCT). Improvements in patient outcomes and satisfaction can be realized when laboratory results are made available in real-time at the patients’ point-of-care. Yet, POCT tends to be siloed and often is not managed by the lab. To meet the evolving needs of healthcare, laboratories need to actively manage POCT. To do so effectively, lab managers who oversee POCT can benefit from a data management and connectivity solution that integrates POCT results into the patient’s chart and eases the challenges of POCT management.
Shift in Healthcare Renews Focus on Integrated POCT
The U.S. healthcare system is undergoing tremendous change, focusing on improved population health, better patient outcomes, and more patient engagement—all at affordable and reasonable costs. With this new focus, point-of-care testing (POCT) is becoming the great boom because of its tremendous potential in value-based care, specifically in situations where a rapid turnaround time
(TAT) can have a profound impact on downstream costs and outcomes. The biggest advantage to POCT is that providing faster access to test results can expedite speed of diagnosis and subsequent
treatment. For example, POCT in the right situations (such as an urgent care location or emergency department) can reduce unnecessary hospital admissions or imaging.
POCT is associated with increased patient satisfaction, an important element of the modern healthcare landscape. This is driving growth in the POCT market. POCT typically requires a much smaller sample volume and this can be significant for certain patient populations like neonatal or ICU patients to avoid iatrogenic anemias. The technology behind the devices and the ability to electronically integrate the results is becoming more reliable and more affordable, which is also driving adoption.
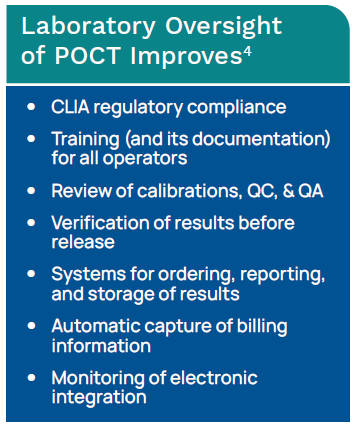
POCT Management Challenges
In order to achieve the real-time benefits associated with POCT, not only does the test have to be performed at the point of care, but the results have to be electronically integrated into the patients’ charts and immediately available to the care team. When this is the case, POCT can improve the efficiency of providers and potentially improve patient outcomes.1 However, management of POCT dispersed throughout a healthcare organization presents many challenges. For example, POCT often takes place in silos within a larger healthcare delivery network and may not be under the laboratory’s management.
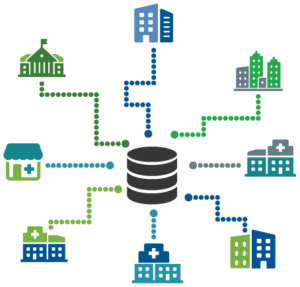 POCT Integration from Decentralized Testing Locations
POCT Integration from Decentralized Testing Locations
Because POCT sites can be widely scattered across a healthcare campus, it is important to have a robust POCT management system capture important testing information and manage regulatory compliance.2 Ideally, the POCT program can be efficiently and effectively monitored from a remote location. In reality, few healthcare organizations have been able to successfully integrate their POCT into their EHR. POCT decentralization is one of the factors that contributes to the fact that only 10% of POCT are electronically integrated.3 The full value of POCT is only seen when those results are immediately accessible in the patients’ EHR.
Management Challenge: Operator Certifications
Not only do healthcare organizations performing POCT need those results integrated to expedite TAT, but in a large facility with hundreds of devices and thousands of operators, POCT operator certification can be quite a challenge. Tracking a large number of operator training and certification dates can be better managed with assistance from a POCT management system. Regulatory requirements directly associated with POCT are increasingly making it difficult to manually monitor a comprehensive POCT program.4
Often POCT operators are not trained as laboratorians, so they may not see the reason behind certain seemingly tedious tasks (QC, QA, maintenance, etc.). When POCT falls in with other lab testing so that it is viewed as a laboratory extension, it is more readily accepted that POCT results should be included in any overall interface decisions related to lab testing.4
 Device Tracking, QC, & Calibration Verification
Device Tracking, QC, & Calibration Verification
Along with keeping up with a multitude of operator certifications, large POCT operations have a large number of POCT devices that must be tracked. QC and calibration verification must be monitored for each device to assure quality testing and meet regulatory requirements. Having a POCT management system that allows the Point-of-Care Coordinator (POCC) to remotely track devices, review QC, and document calibration verification can be useful in large POCT operations.
Billing Challenges
In addition, often the processes in place for POCT billing capture are either poorly organized in a manual system or just not billed for at all. The fact that most POCT is not electronically captured is the cause of substantial loss of revenue that if properly collected could offset the cost of the POCT.4
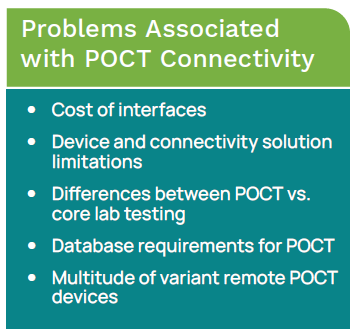
History of POCT Connectivity
Two decades ago, there was essentially no electronic data management for POCT. Test results and related information were manually recorded in the patients’ paper charts or on log sheets located near the testing area. To manage POCT, the POCC would have to physically visit each testing location to review documentation.2 Technology to enable real-time remote management of POCT data was unavailable. Additionally, there were no standards that required POCT devices be designed for connectivity.2 Historically, laboratory information systems (LIS) had not been developed to include management of POCT data.
POCT: From Bedside Glucose to Interfaces
Bedside glucose testing is by far the most widespread and largest volume in the POCT market. These were the first devices developed for the POCT market. In many hospitals, this involves hundreds of devices and thousands of operators. First generation POCT devices developed in the 1990s involved the POCC transporting a laptop into each POCT location and downloading patient results each month. Later, devices were developed that were kept in the patient testing locations cradled in their own docking stations. Results from the devices were downloaded each time the device was docked. This potentially involved a bi-directional interface that allowed for reagent lot numbers and QC data to be downloaded to the devices, and it could be interfaced to the billing system or LIS.2 Next came the ability to interface devices from different manufacturers, followed by real-time wireless connectivity with bi-directional communication to wireless-enabled devices.2
POCT Connectivity Standards
In 2000, the Connectivity Industry Consortium was formed with the goal of developing POCT connectivity standards. These standards evolved into the Clinical and Laboratory Standards Institute (CLSI) POCT1-A.2 The connectivity standard greatly improved the quality of POCT. The intent of the standard was to work toward a plug and play environment for POCT connectivity, where devices are easily interfaced to the LIS, EHR, and HIS.
 The standards also opened the door for the development of POCT management systems capable of connecting multiple devices from various manufacturers, eliminating the need for a computer for each POCT device. CLSI now has a consensus committee that oversees the development of ongoing POCT standards.3
The standards also opened the door for the development of POCT management systems capable of connecting multiple devices from various manufacturers, eliminating the need for a computer for each POCT device. CLSI now has a consensus committee that oversees the development of ongoing POCT standards.3
Today there are many devices capable of reducing or eliminating operator and analytical errors, and there are POCT management systems available that enable automatic electronic flow of data from the POCT devices to the LIS and EHR, as well as providing remote access to QC and operator certification data. While many POCT devices can be directly interfaced to the LIS, most are connected to the LIS through proprietary data management systems (DMSs).2
In years past, manufacturers of POCT devices focused on improving analytical performance. Laboratories would integrate POCT solutions without concern over how the results would be integrated. Now, these manufacturers must also provide up-to-date connectivity and informatics solutions inherent to the devices in order for the POCT devices to be relevant. Savvy laboratories are looking for POCT solutions capable of data integration and IT solutions that encompass all aspects of laboratory information management.4
POCT Case Study: Healthcare Organization in Nebraska
In discussing the transition to using a POCT connectivity solution, the Laboratory and Pathology Operations Manager at a large multi-specialty healthcare organization in Nebraska feels that one of the biggest challenges involved in the oversight of POCT is the fact that testing is commonly performed by a multitude of non-laboratory trained personnel. And while these may be top employees, it can be difficult to train them about the importance of proper QC, documentation, and other laboratory-focused regulatory requirements. The ability to remotely manage certifications and QC can be of great value to a busy manager who has the added task of POCT oversight.



