In today’s healthcare market, laboratories are expected to support value-based care models by achieving maximum productivity with a focus on safety and patient outcomes. Is your laboratory on target to thrive in the evolving landscape? Are you using all the tools and features included in your LIS to their maximum capabilities?
There is a perfect storm—the transition to value-based care, industry consolidation, workforce shortages, technology advances—demanding, and making it possible, that today’s laboratories find ways to increase their productivity with less staff. The most successful laboratories are thinking along this line, focusing on adapting to change and leveraging as many tools as possible to enable their maximum contribution.
By now, nearly every sustainable laboratory of significant size has a laboratory information system (LIS). An LIS is an invaluable tool to support laboratory workflow, streamline processes, and automate decisions. However, there may be features or tools in your LIS that are not being taken advantage of or have been overlooked in the past. Now is the time to be cognizant of your laboratory’s contribution to patient care and to make sure you are on the road to future success by using every tool at hand to its full capability.
Healthcare & Laboratory Industry Climate
In pursuit of the Triple Aim goals of improving patient experience, ensuring better patient outcomes, and reducing overspending, our healthcare system is on the path to value-based care. Consolidation and mergers continue to shape the healthcare landscape, with growing pains exacerbated by the healthcare professionals workforce shortage. Laboratories have the added stress created by the Protecting Access to Medicare Act of 2014 (PAMA).
Industry Consolidation Continues

In response to the shift to value, the consolidation taking place across the healthcare industry is needed to create the economy of scale necessary for healthcare organizations (HCOs) to achieve greater efficiency and subsequently lower costs. Consolidation of large hospital health networks and the closure of provider-run groups continues throughout the healthcare industry and impacts related laboratories. Between 2012 and 2016, hospital-employed physicians increased by more than 63%.1 Healthcare consolidation presents new challenges for laboratories, with some being forced to close their doors when their services are taken over by an in-house hospital laboratory as part of a merger.
PAMA Takes a Toll
In addition, PAMA is in the process of enacting significant changes to the Medicare clinical laboratory fee schedule (CLFS) and creating a significant challenge for laboratories that are required to report their data. PAMA has already prompted a significant reduction in payment for approximately 75% of laboratory tests that are billed to the Centers for Medicare & Medicaid Services (CMS). CMS estimated the first-year reimbursement cuts to approximate $670 million, a 10% decrease from the previous fee schedule, with more cuts expected each year through 2021. Some suggest that PAMA will cause more industry consolidation as the larger reference laboratories (LabCorp and Quest Diagnostics) continue to buy smaller laboratories.2
Growing Shortage of Laboratory Professionals
Another significant industry factor is the continuing decline in the number of qualified medical laboratory scientists (MLSs). According to the Bureau of Labor Statistics (BLS), an estimated 335,700 MLSs and technicians were employed in the United States in 2016. The BLS predicts the need for a 13% average increase in this career between 2016 and 2026—nearly double the average increase in all occupations. The U.S. Department of Health and Human Services projects a 22% increase in demand for MLSs and technicians between 2012 and 2025.
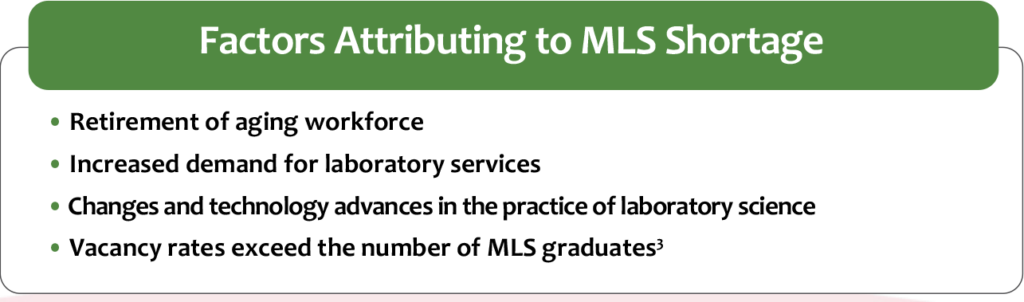
There is a crucial need for qualified MLSs, yet schools are scarce, not matching the demand for new scientists as the older generation reaches retirement. The industry is experiencing an increase in non-certified individuals performing low- to high-complexity tests and an increased rate of laboratory staff taking on more responsibilities. Figure 2 lists several factors that are contributing to the MLS workforce shortage. The lack of recognition and support of strong professional standards attributes to problems with employee retention and satisfaction. Figure 3 defines more challenges to retaining qualified laboratory personnel.
As a result of the shortage of qualified, laboratory-trained staff, some laboratories resort to hiring employees with bachelor’s degrees in biology or chemistry and training them on the job. Other healthcare professions have not reached this level of desperation because of professional standards requirements. This situation can negatively impact the overall quality of laboratory services and is a potential threat to patient safety.3

What Successful Labs Are Doing to Adapt & Thrive
Savvy laboratories are attempting to adapt to these changes by finding ways to increase their productivity and their overall value to their organizations. By being aware of the healthcare landscape, laboratories can actively plan to focus on staff retention and satisfaction. They can promote greater laboratory stewardship to secure their place in the diagnostic continuum. Additionally, laboratory professionals must use the tools they already have effectively. For example, let the LIS do the automated work it was meant for so you can focus on the tasks that require skilled technologists. Then, you can find the time to catch those erroneous results or properly troubleshoot and document an analyzer issue that you barely had time to deal with before.
Recruitment & Retention Efforts
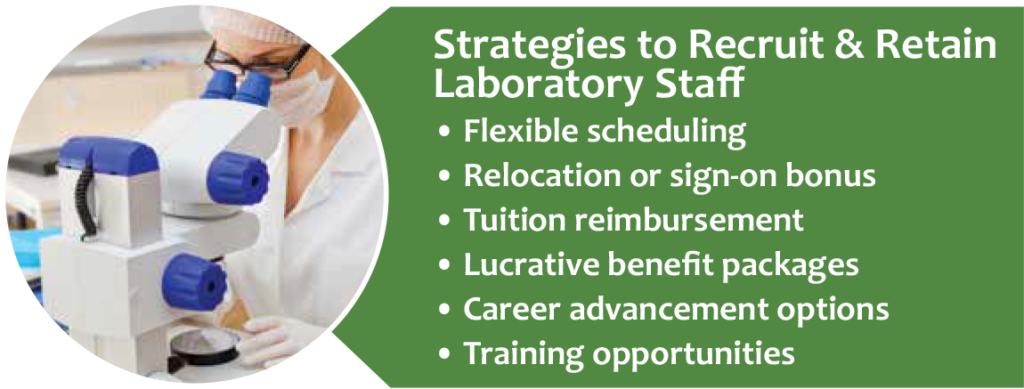
With competition among laboratories to hire and retain skilled MLSs, top organizations are finding innovative ways to recruit and retain the best workers (see Figure 4). Alongside competitive salaries and benefits, opportunities for career advancement, continuing education, and tuition reimbursement are attractive to new employees. Recently graduated students may be interested in help paying off student loans. For labs in areas where it is especially hard to hire new employees, a sign-on bonus can be an added incentive.
The most innovative laboratories are developing creative recruitment and retention plans. For example, Memorial Sloan Kettering Cancer Center has a Laboratory Scholars Program that offers laboratory technologist training to employees from other departments within the organization.4 TriCore Reference Laboratories has a similar Histology Apprenticeship Program that offers histotechnology training to interested TriCore employees.4 Using their nursing department’s example as a guide, the University of Vermont Medical Center developed an effective Lab Preceptor Program that trains laboratorians to be successful teachers for new hires.5 Accepting this responsibility offers a way to move up the career ladder and improves job satisfaction.
Laboratory Stewardship: Taking Full Responsibility
The idea of laboratory stewardship, in which a laboratory takes full responsibility for lab testing from start to finish, is important regarding contribution level, efficiency, and productivity. Laboratory stewardship moves beyond tracking laboratory utilization and takes the lab formulary to the next level, focusing on making sure appropriate tests are ordered and properly interpreted. The idea is to think beyond reporting the test result to downstream healthcare processes related to lab testing. Laboratorians can help providers in determining best test selection, performance, and interpretation. Laboratory stewardship means the laboratory takes responsibility for the use of lab testing, from order through interpretation to analytics. Achieving Standardization for Greater Consistency Laboratory standardization, including uniformity in test methodologies and procedures, falls under good laboratory stewardship and is one way to decrease the burden of training employees. Standardizing laboratory practices using evidence-based guidelines can improve efficiency, patient satisfaction, quality of care, and overall clinical operations.6 In a large, consolidated delivery network, patients benefit from consistency in laboratory testing throughout the organization. Differences in testing methodologies can be confusing and potentially lead to misinterpreted results. When testing policies, procedures, and methodologies are standardized, manageability, quality, and efficiency improve due to decreased redundancy and errors. In addition, EHR and LIS setup becomes uniform across locations, reducing opportunities for misinterpretation.
As an example, Atrium Health in Charlotte, North Carolina, has spent the last several years working to standardize its test menu and methodologies, reference ranges, and analyzers and test platforms across 10 laboratory testing sites within the organization. By standardizing these systems, Atrium expects to see improvements in workflow efficiency, turnaround times (TATs), staff productivity, quality, and overall value contribution.7
The trend toward decentralized testing—offering lab testing at the point of patient care—is expected to continue and can also be improved upon with standardization. Systems with locations spread over large geographic areas may be better served by a decentralized structure, where each facility manages its own lab operation.
However, with decentralized testing, it becomes even more important to standardize products and processes. Standardized policies and procedures help ensure that all team members are following the same processes, and every facility is conducting testing in an accurate, efficient, and patient-minded manner. Additionally, HCOs can achieve significant savings by reviewing the products each facility is purchasing. They can then set purchasing standards to obtain the most cost-effective products that provide the best patient outcomes.
Leveraging Automation for Processing Prowess
Another move to decrease costs and address the workforce shortage is implementation of a laboratory automation line. The idea is to replace tedious, manual processing tasks with robotics, and save the complex, evaluative tasks for the laboratory scientists. In addition to freeing staff for other duties, various forms of laboratory automation can:
- decrease repetitive injuries
- improve TATs
- improve cost effectiveness (by speeding process time)
- eliminate human error
- improve tube tracking ability
- improve job satisfaction
Maximizing Your LIS as a Productivity Tool
A laboratory’s LIS is arguably its most powerful tool, especially when it is leveraged to its maximum potential. Used properly, the LIS can greatly improve laboratory productivity, efficiency, and safety. By careful configuration of analyzer and EHR interfaces, specific rules, tracking features, and automated reporting and metrics, the LIS makes the job of laboratory professionals easier, allowing their expertise to be focused on tasks that require human interaction.
Workflow Management Improvements
As part of an effective laboratory workflow, instrument and system integration provides an automatic and seamless flow of data, allowing your laboratory to improve patient care by reducing errors, saving time, and speeding up the delivery of results. Connectivity is a critical component in both centralized and decentralized labs. Laboratory analyzers should be connected through the LIS to your EHR, allowing test results to be stored and shared across facilities in an automated manner.
In addition, many LISs include audit trails and sample tracking features that provide laboratories the tools to organize and track laboratory samples and specimens throughout the testing continuum. A sample tracking tool can organize and track the storage of laboratory samples and specimens to allow easier retrieval. Rather than searching through tubes or slides to review dates, you can easily track expired items in the sample tracking system, and the system can alert users when items have reached their expiration. By automating these features, your laboratory has the required documentation to track a specimen from start to finish for a regulatory inspection.
Rules That Automate Processes & Decisions
Throughout the entire testing process, from pre-analytical to post-analytical (see Figure 5), robust LISs can be set up to automatically trigger rules that make life in the laboratory much easier. In support of laboratory stewardship, the LIS can be a tool that allows MLSs to work closely with ordering providers to eliminate obsolete or non-value added testing and avoid unnecessary test combinations, contributing to overall improvements in the quality of patient care. Rules can virtually eliminate the ever-present “sticky notes” that line the processing and bench tech areas (see Figure 6). Configurable rules improve workflow efficiency and error-proofing by aiding in decision-making throughout the total testing process. This ensures the consistent execution of procedures and increases the productivity of your laboratory.
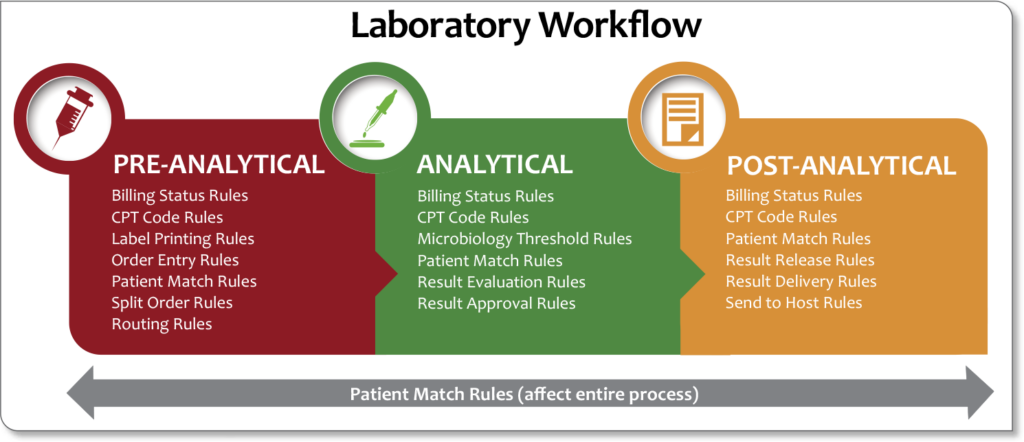
For example, pre-analytical rules can be implemented to:
- eliminate duplicate orders or tests not appropriate for a patient
- split an order based on sample handling, batch testing, or other requirements
- route tests to outside labs based on reimbursements
- automatically print labels when reflex testing is indicated
Analytical rules can:
- automate reflex or cascade testing
- auto-schedule QC and auto-verify acceptable QC
- prevent release of results without acceptable QC
- schedule reminder messages for maintenance, linearity testing, or other “to-do” items

Post-analytical rules can be implemented to:
- automate venipuncture charges
- consolidate or change billing codes based on testing conditions
- schedule courtesy copies of results
- automate fax and email of invoices and requisitions
- automate report delivery based on certain conditions
- auto-verify “normal” results
Autoverification: A Tremendous Time-saver
With the tremendous squeeze on laboratories to continually do more with less coming to a peak with healthcare reform, labs must respond to these challenges to improve quality, reduce costs, and simplify processes. Autoverification is one of the key tools to achieve these goals. Depending on your lab menu and patient population, 40% to 80% of results could qualify for autoverification. Autoverification rules provide consistent test reporting with dramatic improvements in TAT and reduction in error rates.
Autoverification of results is one of the absolute most time-saving, beneficial undertakings a laboratory can implement. Some laboratorians may struggle with letting go of reviewing instrument printouts and verifying the results transmitted properly into the LIS prior to approving for release, but once successful autoverification rules are in place, the time-savings are clearly worth the change and effort. If your LIS has the technology, use it!
Washington Health System Uses Autoverification for Up to 80% of Hematology Results
Washington Health System, a longtime Orchard Software customer, uses autoverification as a productivity booster. LIS Coordinator Chuck Kulla and LIS Specialist Carrie Lough agree that setup of autoverification in hematology was relatively simple. “Autoverification setup was easy in hematology because the flags are defined and the rules are easy to write. We autovalidate about 80% of results in hematology,” explains Lough. In addition, some chemistry tests and blood cultures are set up to auto-verify, saving tech time and speeding the time to result delivery.
Autoverification Defined
The Clinical & Laboratory Standards Institute (CLSI) defines autoverification as “the automated actions performed by a computer system related to the release of results to the medical record using criteria and logic established, documented, and tested by the medical staff of the laboratory.” In other words, autoverification is a laboratory process in which acceptable patient result parameters are defined for results generated from interfaced instruments and sent to the LIS. Results that fall within the defined autoverification parameters automatically release into the EHR without any additional laboratory staff review or intervention. Results that are not within the defined parameters are saved for technologist review prior to reporting.
Start Small
If autoverification seems like a daunting project, take the process one small step at a time. For example:
- Select one test on your laboratory menu to begin autoverification.
- Determine your acceptable parameters or result evaluation monitors.
- Implement the applicable LIS rules.
- Test the new autoverification rules on “test” patients.
- Test the new autoverification rules on real patients.
- Turn on autoverification rules for that one test only.
- Monitor closely until you have verified that a sufficient volume of that test has properly transmitted into the EHR.
The results being released are still in your control. The rules protect any results you have flagged as needing review from inadvertently being released. You can tweak the rules as you go through the process and gain more experience.
Autoverification Steps
These steps are based on Orchard® Harvest™ and will vary from LIS to LIS; however, the basic premise should be similar.
- Do your research. There are numerous resources available. Consider using the CLSI standard, “AUTO10 – Autoverification of Clinical Laboratory Test Results; 1st Edition,”8 although there are many free resources available. CLSI also has a new autoverification document available: “AUTO15-Ed1 – Autoverification of Medical Laboratory Results for Specific Disciplines, 1st Edition.”
- Review your current result approval workflow and use it to create a basic autoverification policy that fits the specific testing menu and volume of your laboratory. Remember that it can be updated as you go along, but it can be helpful to find a starting point to refer to as you go through the process.
- Determine the acceptable autoverification range. Your acceptable autoverification range defines the acceptable result limits for a lab test that can be released without technologist review. Pull LIS reports of previous results to analyze for each test and determine cutoff points where it is necessary for technologist review. Below are some factors to keep in mind as you determine your appropriate range
- analyzer linearity restrictions
- analyzer auto-dilution procedures
- critical values
- delta checks
- QC requirements
- instrument flags and how they interact with your interface
- rerun policy
- reference ranges, including age and gender specific
- integrity checks
- Create autoverification rules in your test system. Create “test” lab tests and order choices that duplicate your real tests and order choices and use those to create autoverification rules.
- Once you have successfully verified a representative number of “test” patients, create the autoverification rules in your live system while still reviewing result transmissions to verify accuracy. Your review should include enough volume that several tests fail to auto-verify. Ideally, you would like to encounter each failed scenario and ensure its proper function. Create a spreadsheet to keep track and document the test samples.
- Train staff throughout the process.
- Verify annually.
More Autoverification Resources
- Krasowski MD, Davis SR, Drees D, et al. Autoverification in a core clinical chemistry laboratory at an academic medical center. J Pathol Inform. 2014;5(1). doi:10.4103/2153-3539.129450. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4023033/.
- Marquardt B. A step-by-step process to 95% autoverification. CAP Today. https://www.captodayonline.com/step-by-step-autoverification/. Published December 2015.
- Shih M, Chang H, Tien N, Hsiao C, Peng C. Building and validating an autoverification system in the clinical chemistry laboratory. Laboratory Medicine. 2011; 42(11): 668–73. doi: 10.1309/LM5AM4IIXC4OIETD. https://doi.org/10.1309/LM5AM4IIXC4OIETD.
Using Boolean Logic & Flow Charts to Develop Autoverification Rules
Boolean Logic
The basic concept behind autoverification follows Boolean logic, which is a set of logic rules that trigger in a specified order to provide consistent results. The most common operators are AND, OR, and NOT.
Flow Chart Logic
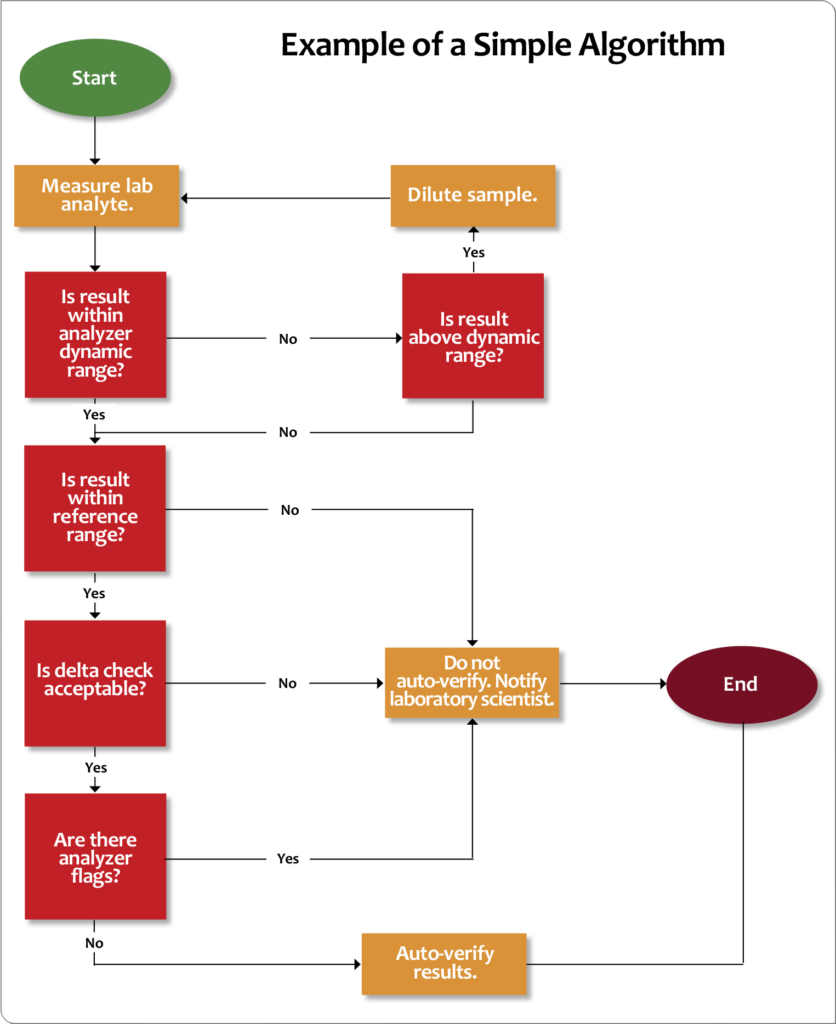
Other tools that can help determine autoverification rules are flow charts, such as the example depicted in Figure 7. Using flow chart logic, go through your autoverification list and answer “Yes” or “No” to each scenario (e.g., review dynamic range, normal range, delta checks, etc.). If the answers are all “Yes,” the result can be auto-approved. If not, other actions must be taken according to laboratory procedures.
Autoverification Regulatory Tips
All CLIA and Joint Commission requirements through pre-analytical, analytical, and post-analytical testing phases will apply to your autoverification processes. The College of American Pathologists (CAP) also has an autoverification checklist. Here is a list of some basic regulatory requirements.
- The laboratory director must approve and sign the autoverification policy and procedures.
- Verification must be performed initially and annually.
- You must have a process to ensure acceptable QC for auto-verified tests.
- The laboratory audit trail must indicate test results that are auto-verified, as well as date/time stamp of autoverification.
- For any significant changes in your analytical procedures that affect autoverification (e.g., analyzer change, specimen handling requirements, etc.), you must repeat the verification process.
- Your QA program should include evaluation of the autoverification process. It is good practice to maintain autoverification rule traceability so that you can look back and determine what rules were in place during each testing period.9
- Your autoverification setup should include delta checks.
- Your autoverification policy needs to include a rapid suspension procedure so that in the event of a problem, staff can turn off autoverification.
Metrics That Are a Must
Laboratories handle an enormous amount of data, much of it vital to patient diagnosis and treatment decisions. Data that lives in your LIS should be made readily available to your organization to help achieve quality goals, promote population health management, and close care gaps. Use your LIS to set up automated reports to track laboratory metrics on a consistent basis. Metrics such as utilization monitoring, productivity, and TAT tracking can be set up to automatically report to the desired individuals at a specified time and location (see Figure 8). This automated process can save valuable time and help the flow of communication.
business metrics, such as utilization or productivity
monitoring, or turnaround times.
More LIS Productivity Tips: Orchard’s Advanced User Training
We know that the better trained you are to use your LIS, the more you can take advantage of each feature to improve your laboratory’s productivity and efficiency. To maximize your understanding of the many features in our LISs, Orchard offers various training options.

Our customer training courses are held in the Training Center at Orchard Software (see Figure 9), located north of Indianapolis in Carmel, Indiana. These courses give you hands-on experience with the latest new features and help you get more from existing features, maximizing your efficiency with tips and shortcuts.
The majority of these courses are accredited through the American Society for Clinical Laboratory Science (ASCLS) Professional Acknowledgment for Continuing Education (P.A.C.E.®) program. In addition, you have an opportunity to exchange ideas with other users and Orchard employees. Some customers find it beneficial for budgeting purposes to select an annual service agreement that includes a yearly visit to Carmel for advanced training. For more information, please contact your Account Manager at (800) 856-1948.
Reference List
- Peck AD. Healthcare mergers, physician consolidation, and increased healthcare utilization expected to increase medical cost by 6% in 2019. Dark Daily. https://www.darkdaily.com/healthcare-mergers-physician-consolidationand-increased-healthcare-utilization-expected-to-increase-medical-cost-by-6-in-2019/. Published November 23, 2018.
- Fong T. The PAMA effect: Consolidation of clinical labs expected as legislation set to take effect. 360 Dx. https://www.360dx.com/regulatory-news/pama-effect-consolidation-clinical-labs-expected-legislation-set-takeeffect#.XP5yI4hKiUk. Published May 30, 2017.
- ASCLS. Addressing the clinical laboratory workforce shortage. 2018. http://www.ascls.org/images/publications/Clinical_Laboratory_Workforce_FINAL_20180824.pdf.
- Newitt VN. Too few technologists: labs take inventive steps. CAP Today. https://www.captodayonline.com/too-few-technologists-labs-take-inventive-steps/. Published April 2019.
- Newitt VN. Not a fan of training new hires? Lab finds a fix. CAP Today. https://www.captodayonline.com/not-afan-of-training-new-hires-lab-finds-a-fix/. Published February 2019.
- Yu E. Lab standardization in the era of big healthcare networks. Clinical Laboratory News. https://www.aacc.org/publications/cln/articles/2017/september/lab-standardization-in-the-era-of-big-healthcare-networks. Published September 2017.
- McBride M. Innovative clinical laboratories of hospitals and health systems now working to standardize and rationalize lab tests and lab services. Dark Daily. https://www.darkdaily.com/innovative-clinical-laboratoriesof-hospitals-and-health-systems-now-working-to-standardize-and-rationalize-lab-tests-and-lab-services-92418/. Published September 24, 2018.
- Clinical and Laboratory Standards Institute. AUTO10-A: Autoverification of clinical laboratory test results; approved guideline. 2006:26(32). https://clsi.org/standards/products/automation-and-informatics/documents/auto10/.
- Tate A. Auto-verification rules—now what? MLO. https://www.mlo-online.com/information-technology/lis/article/13017229/autoverification-rulesnow-what. Published January 23, 2019.

